The role of the amygdala in ictal central apnea: insights from brain MRI morphometry
- PMID: 37936526
- PMCID: PMC10791031
- DOI: 10.1002/acn3.51938
The role of the amygdala in ictal central apnea: insights from brain MRI morphometry
Abstract
Objective: Ictal central apnea (ICA) is a frequent correlate of focal seizures, particularly in temporal lobe epilepsy (TLE), and regarded as a potential electroclinical biomarker of sudden unexpected death in epilepsy (SUDEP). Aims of this study are to investigate morphometric changes of subcortical structures in ICA patients and to find neuroimaging biomarkers of ICA in patients with focal epilepsy.
Methods: We prospectively recruited focal epilepsy patients with recorded seizures during a video-EEG long-term monitoring with cardiorespiratory polygraphic recordings from April 2020 to September 2022. Participants were accordingly subdivided into two groups: patients with focal seizures with ICA (ICA) and without (noICA). A pool of 30 controls matched by age and sex was collected. All the participants underwent MRI scans with volumetric high-resolution T1-weighted images. Post-processing analyses included a whole-brain VBM analysis and segmentation algorithms performed with FreeSurfer.
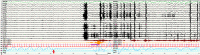
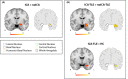
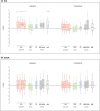
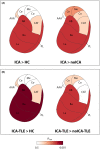
Results: Forty-six patients were recruited (aged 15-60 years): 16 ICA and 30 noICA. The whole-brain VBM analysis showed an increased gray matter volume of the amygdala ipsilateral to the epileptogenic zone (EZ) in the ICA group compared to the noICA patients. Amygdala sub-segmentation analysis revealed an increased volume of the whole amygdala, ipsilateral to the EZ compared to controls [F(1, 76) = 5.383, pFDR = 0.042] and to noICA patients ([F(1, 76) = 5.383, pFDR = 0.038], specifically of the basolateral complex (respectively F(1, 76) = 6.160, pFDR = 0.037; F(1, 76) = 5.121, pFDR = 0.034).
Interpretation: Our findings, while confirming the key role of the amygdala in participating in ictal respiratory modifications, suggest that structural modifications of the amygdala and its subnuclei may be valuable morphological biomarkers of ICA.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
E. Micalizzi has served as a paid consultant for Angelini Pharma. F. Villani has served as a paid consultant and received support from Angelini Pharma, UCB Pharma, EISAI, Lusofarmaco, Jazz Pharma, Bial.S. Meletti has served as a paid consultant and received support from the Ministry of Health (MOH), UCB, GW, Jazz pharmaceuticals and EISAI. A.E. Vaudano has served as a paid consultant for Angelini Pharma. The other authors report no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

