Incidence of hepatitis C virus infection in the prison setting: The SToP-C study
- PMID: 37936544
- PMCID: PMC10952254
- DOI: 10.1111/jvh.13895
Incidence of hepatitis C virus infection in the prison setting: The SToP-C study
Abstract
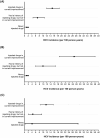
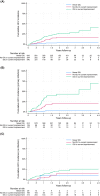
People in prison are at high risk of HCV given high injecting drug use prevalence. This study evaluated HCV incidence and associated injecting drug use characteristics in prison. The SToP-C study enrolled people incarcerated in four Australian prisons. Participants were tested for HCV at enrolment and then every 3-6 months (October-2014 to November-2019). Participants eligible for this analysis included those at-risk of HCV primary infection (anti-HCV negative) or re-infection (anti-HCV positive, HCV RNA negative) with follow-up assessment. A total of 1643 eligible participants were included in analyses (82% male; median age 33 years; 30% injected drugs in prison; 1818 person-years of follow-up). Overall HCV incidence was 6.11/100 person-years (95%CI: 5.07-7.35), with higher rate of re-infection (9.34/100 person-years; 95%CI: 7.15-12.19) than primary infection (4.60/100 person-years; 95%CI: 3.56-5.96). In total population (n = 1643), HCV risk was significantly higher among participants injecting drugs in prison [vs. no injecting; adjusted hazard ratio (aHR): 10.55, 95%CI: 5.88-18.92), and those who were released and re-incarcerated during follow-up (vs. remained incarcerated; aHR: 1.60, 95%CI: 1.03-2.49). Among participants who injected recently (during past month, n = 321), HCV risk was reduced among those receiving high-dosage opioid agonist therapy (OAT), i.e. methadone ≥60 mg/day or buprenorphine ≥16 mg/day, (vs. no OAT, aHR: 0.11, 95%CI: 0.02-0.80) and increased among those sharing needles/syringes without consistent use of disinfectant to clean injecting equipment (vs. no sharing, HR: 4.60, 95%CI: 1.35-15.66). This study demonstrated high HCV transmission risk in prison, particularly among people injecting drugs. High-dosage OAT was protective, but improved OAT coverage and needle/syringe programmes to reduce sharing injecting equipment are required.
Keywords: HCV; cohort study; correctional facilities; intravenous substance abuse; opiate substitution treatment.
© 2023 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
GJD is a consultant or adviser for, and has received research grants from, AbbVie, Abbot Diagnostics, Gilead Sciences, Bristol Myers Squibb, Cepheid, GlaxoSmithKline, Merck, Janssen and Roche. ARL is a consultant/adviser and has received investigator‐initiated research grants from Gilead, AbbVie and Bristol‐Myers Squibb. JG is a consultant or adviser for, and has received research grants from AbbVie, bioLytical, Camurus, Cepheid, Gilead Sciences, Hologic and Indivor, and has received honoraria from AbbVie, Cepheid and Gilead Sciences. NM has received research grants from AbbVie and Gilead Sciences. PV has received research grants from Gilead Sciences. Other co‐authors had none to declare.
Figures
References
-
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553‐562. - PubMed
-
- Penal Reform International . Global Prison Trends 2022. Penal Reform International; 2022.
-
- Degenhardt L, Webb P, Colledge‐Frisby S, et al. Epidemiology of injecting drug use, prevalence of injecting‐related harm, and exposure to behavioural and environmental risks among people who inject drugs: a systematic review. Lancet Glob Health. 2023;11(5):e659‐e672. - PubMed
-
- Sander G, Shirley‐Beavan S, Stone K. The global state of harm reduction in prisons. J Correct Health Care. 2019;25(2):105‐120. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

