Identifying Mitral Valve Prolapse at Risk for Arrhythmias and Fibrosis From Electrocardiograms Using Deep Learning
- PMID: 37936601
- PMCID: PMC10629907
- DOI: 10.1016/j.jacadv.2023.100446
Identifying Mitral Valve Prolapse at Risk for Arrhythmias and Fibrosis From Electrocardiograms Using Deep Learning
Abstract
Background: Mitral valve prolapse (MVP) is a common valvulopathy, with a subset developing sudden cardiac death or cardiac arrest. Complex ventricular ectopy (ComVE) is a marker of arrhythmic risk associated with myocardial fibrosis and increased mortality in MVP.
Objectives: The authors sought to evaluate whether electrocardiogram (ECG)-based machine learning can identify MVP at risk for ComVE, death and/or myocardial fibrosis on cardiac magnetic resonance (CMR) imaging.
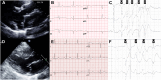
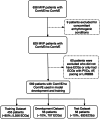
Methods: A deep convolutional neural network (CNN) was trained to detect ComVE using 6,916 12-lead ECGs from 569 MVP patients from the University of California-San Francisco between 2012 and 2020. A separate CNN was trained to detect late gadolinium enhancement (LGE) using 1,369 ECGs from 87 MVP patients with contrast CMR.
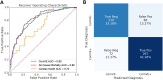
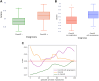
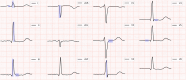
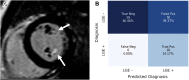
Results: The prevalence of ComVE was 28% (160/569). The area under the receiver operating characteristic curve (AUC) of the CNN to detect ComVE was 0.80 (95% CI: 0.77-0.83) and remained high after excluding patients with moderate-severe mitral regurgitation [0.80 (95% CI: 0.77-0.83)] or bileaflet MVP [0.81 (95% CI: 0.76-0.85)]. AUC to detect all-cause mortality was 0.82 (95% CI: 0.77-0.87). ECG segments relevant to ComVE prediction were related to ventricular depolarization/repolarization (early-mid ST-segment and QRS from V1, V3, and III). LGE in the papillary muscles or basal inferolateral wall was present in 24% patients with available CMR; AUC for detection of LGE was 0.75 (95% CI: 0.68-0.82).
Conclusions: CNN-analyzed 12-lead ECGs can detect MVP at risk for ventricular arrhythmias, death and/or fibrosis and can identify novel ECG correlates of arrhythmic risk. ECG-based CNNs may help select those MVP patients requiring closer follow-up and/or a CMR.
Keywords: artificial intelligence; computers; echocardiography; valvular heart disease.
Conflict of interest statement
This work was supported by the UCSF Cardiology Innovation Award and by the National Institutes of Health NHLBI R01HL153447 (Drs Delling and Tison) and NHLBI K23HL135274 (Dr Tison). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr Delling has received consultant fees from Zogenix. Dr Tison has previously received research grants from General Electric, Janssen Pharmaceuticals, and Myokardia. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


Comment in
-
Artificial Intelligence-Enhanced Electrocardiographic Analysis in Mitral Valve Prolapse: Hunting for Zebras.JACC Adv. 2023 Jul 27;2(6):100443. doi: 10.1016/j.jacadv.2023.100443. eCollection 2023 Aug. JACC Adv. 2023. PMID: 38939427 Free PMC article.
References
-
- Freed L.A., Levy D., Levine R.A., et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. - PubMed
-
- Sriram C.S., Syed F.F., Ferguson M.E., et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. - PubMed
-
- Nalliah C.J., Mahajan R., Elliott A.D., et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105:144–151. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

