Autologous Stem Cell Collection after Daratumumab, Bortezomib, Thalidomide, and Dexamethasone versus Bortezomib, Cyclophosphamide, and Dexamethasone in Newly Diagnosed Multiple Myeloma
- PMID: 37936633
- PMCID: PMC10626396
- DOI: 10.1159/000529691
Autologous Stem Cell Collection after Daratumumab, Bortezomib, Thalidomide, and Dexamethasone versus Bortezomib, Cyclophosphamide, and Dexamethasone in Newly Diagnosed Multiple Myeloma
Abstract
Introduction: In transplant-eligible, newly diagnosed multiple myeloma (NDMM) patients, autologous peripheral blood stem cell (PBSC) collection is usually pursued after induction therapy. While induction regimens are constantly refined regarding response, their impact on PBSC collection is not fully studied. The inclusion of the anti-CD38 antibody daratumumab into induction therapy significantly improved outcomes for patients with NDMM, e.g., as part of the daratumumab, bortezomib, thalidomide, and dexamethasone (Dara-VTD) protocol. Preliminary data from the phase 3 CASSIOPEIA study proved the efficacy of Dara-VTD. While overall PBSC collection upon addition of daratumumab was reduced in the study population, more detailed analyses on the impact are missing.
Methods: We here report on PBSC mobilization and collection metrics in n = 119 patients with NDMM who underwent induction therapy with bortezomib, cyclophosphamide, and dexamethasone (VCD, n = 61) or Dara-VTD (n = 58).
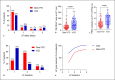
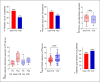
Results: Patient characteristics were well balanced between groups. The Dara-VTD group showed improved response parameters with 66% of patients reaching at least very good partial response versus 54% in the VCD group. Dara-VTD patients exhibited inferior mobilization metrics such as peripheral blood CD34+ cell count at the first leukapheresis (LP) session (65 vs. 106/μL, p = 0.001), median number of LP sessions (2 vs. 1, p = 0.001), and PBSC collection at first LP (5.5 vs. 8.3 × 106/kg body weight [bw], p = 0.001). Utilization of plerixafor was slightly higher after Dara-VTD (33% vs. 21% of patients, p = 0.143). The overall PBSC collection result was significantly lower after Dara-VTD (8.4 vs. 9.6 × 106/kg bw, p = 0.026). 78% and 85% of patients successfully collected 3 transplants with ≥2 × 106 CD34+ cells/kg bw in the Dara-VTD and the VCD groups, respectively.
Conclusion: In summary, Dara-VTD, possibly due to both anti-CD38 antibody and thalidomide exposure, imposes a limitation on PBSC collection which can be only partly overcome by utilization of plerixafor.
Keywords: Daratumumab; Leukapheresis; Multiple myeloma; Stem cell collection; Thalidomide.
Copyright © 2023 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The first authors and all coauthors confirm that there are no potential conflicts of interest to disclose, except the following. Joseph Kauer: Honoraria: AstraZeneca. Sandra Sauer: travel grants or honoraria for presentations for Celgene, BMS, Janssen, Takeda, and Amgen. Katharina Kriegsmann: research funding and honoraria from Sanofi. A.S. received travel grants from Hexal and Jazz Pharmaceuticals, research grant from Therakos/Mallinckrodt, consultant by Janssen-Cilag and BMS, and is co-founder of TolerogenixX Ltd. A.S. is a part-time employee of TolerogenixX Ltd.
Figures
References
-
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335((2)):91–97. - PubMed
-
- Hübel K, de la Rubia J, Azar N, Corradini P. Current status of haematopoietic autologous stem cell transplantation in lymphoid malignancies a European perspective. Eur J Haematol. 2015;94((1)):12–22. - PubMed
-
- Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28((Suppl 4))(iv):52–61. - PubMed
-
- Chute JP. Autologous stem cell transplantation for multiple myeloma underutilized but highly effective. J Natl Cancer Inst. 2019;111((1)):7–8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

