An IKBKE variant conferring functional cGAS/STING pathway deficiency and susceptibility to recurrent HSV-2 meningitis
- PMID: 37937644
- PMCID: PMC10721272
- DOI: 10.1172/jci.insight.173066
An IKBKE variant conferring functional cGAS/STING pathway deficiency and susceptibility to recurrent HSV-2 meningitis
Abstract
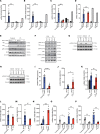
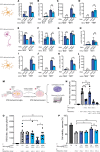
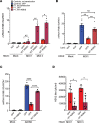
The mechanisms underlying susceptibility to recurrent herpes simplex virus type 2 (HSV-2) meningitis remain incompletely understood. In a patient experiencing multiple episodes of HSV-2 meningitis, we identified a monoallelic variant in the IKBKE gene, which encodes the IKKε kinase involved in induction of antiviral IFN genes. Patient cells displayed impaired induction of IFN-β1 (IFNB1) expression upon infection with HSV-2 or stimulation with double-stranded DNA (dsDNA) and failed to induce phosphorylation of STING, an activation marker of the DNA-sensing cyclic GMP-AMP synthase/stimulator of IFN genes (cGAS/STING) pathway. The patient allele encoded a truncated IKKε protein with loss of kinase activity and also capable of exerting dominant-negative activity. In stem cell-derived microglia, HSV-2-induced expression of IFNB1 was dependent on cGAS, TANK binding kinase 1 (TBK1), and IKBKE, but not TLR3, and supernatants from HSV-2-treated microglia exerted IKBKE-dependent type I IFN-mediated antiviral activity upon neurons. Reintroducing wild-type IKBKE into patient cells rescued IFNB1 induction following treatment with HSV-2 or dsDNA and restored antiviral activity. Collectively, we identify IKKε to be important for protection against HSV-2 meningitis and suggest a nonredundant role for the cGAS/STING pathway in human antiviral immunity.
Keywords: Immunology; Infectious disease; Innate immunity.
Figures



References
-
- Whitley R, et al. Pathogenesis and disease. In: Arvin A, et al., eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; 2007:Chapter 32. - PubMed
-
- Mollaret P. La meningite endothelio-leucocytaire multirecurrente benigne. Syndrome nouveau ou maladie nouvelle? Documents cliniques. [French] Rev Neurol. 1944;76:57–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

