Human strongyloidiasis: complexities and pathways forward
- PMID: 37937980
- PMCID: PMC10732074
- DOI: 10.1128/cmr.00033-23
Human strongyloidiasis: complexities and pathways forward
Abstract
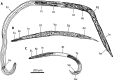
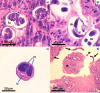
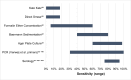
Strongyloidiasis is a World Health Organization neglected tropical disease usually caused by Strongyloides stercoralis, a parasitic worm with a complex life cycle. Globally, 300-600 million people are infected through contact with fecally contaminated soil. An autoinfective component of the life cycle can lead to chronic infection that may be asymptomatic or cause long-term symptoms, including malnourishment in children. Low larval output can limit the sensitivity of detection in stool, with serology being effective but less sensitive in immunocompromise. Host immunosuppression can trigger catastrophic, fatal hyperinfection/dissemination, where large numbers of larvae pierce the bowel wall and disseminate throughout the organs. Stable disease is effectively treated by single-dose ivermectin, with disease in immunocompromised patients treated with multiple doses. Strategies for management include raising awareness, clarifying zoonotic potential, the development and use of effective diagnostic tests for epidemiological studies and individual diagnosis, and the implementation of treatment programs with research into therapeutic alternatives and medication safety.
Keywords: Strongyloides; neglected tropical diseases; soil-transmitted helminth; strongyloidiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Schad GA. 1989. Morphology and life history of Strongyloides stercoralis, p 85–104. In Grove DI (ed), Strongyloidiasis: a major roundworm infection of man. Taylor & Francis Ltd, London.
-
- Forrer A, Khieu V, Schär F, Hattendorf J, Marti H, Neumayr A, Char MC, Hatz C, Muth S, Odermatt P. 2017. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Negl Trop Dis 11:e0005685. doi:10.1371/journal.pntd.0005685 - DOI - PMC - PubMed
-
- Jember TH, Amor A, Nibret E, Munshea A, Flores-Chavez M, Ta-Tang T-H, Saugar JM, Benito A, Anegagrie M. 2022. Prevalence of Strongyloides stercoralis infection and associated clinical symptoms among schoolchildren living in different altitudes of Amhara National Regional State, northwest Ethiopia. PLoS Negl Trop Dis 16:e0010299. doi:10.1371/journal.pntd.0010299 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

