A pig model of chronic hepatitis E displaying persistent viremia and a downregulation of innate immune responses in the liver
- PMID: 37938097
- PMCID: PMC10635601
- DOI: 10.1097/HC9.0000000000000274
A pig model of chronic hepatitis E displaying persistent viremia and a downregulation of innate immune responses in the liver
Abstract
Background: Hepatitis E virus (HEV) is a zoonotic virus transmitted by pig meat and responsible for chronic hepatitis E in immunocompromised patients. It has proved challenging to reproduce this disease in its natural reservoir. We therefore aimed to develop a pig model of chronic hepatitis E to improve the characterization of this disease.
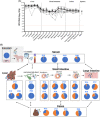
Methods: Ten pigs were treated with a tacrolimus-based regimen and intravenously inoculated with HEV. Tacrolimus trough concentration, HEV viremia, viral diversity, innate immune responses, liver histology, clinical disease and biochemical markers were monitored for 11 weeks post-infection (p.i.).
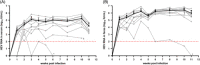
Results: HEV viremia persisted for 11 weeks p.i. HEV RNA was detected in the liver, small intestine, and colon at necropsy. Histological analysis revealed liver inflammation and fibrosis. Several mutations selected in the HEV genome were associated with compartmentalization in the feces and intestinal tissues, consistent with the hypothesis of extrahepatic replication in the digestive tract. Antiviral responses were characterized by a downregulation of IFN pathways in the liver, despite an upregulation of RIG-I and ISGs in the blood and liver.
Conclusions: We developed a pig model of chronic hepatitis E that reproduced the major hallmarks of this disease. This model revealed a compartmentalization of HEV genomes in the digestive tract and a downregulation of innate immune responses in the liver. These original features highlight the relevance of our model for studies of the pathogenesis of chronic hepatitis E and for validating future treatments.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures



References
-
- Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. - PubMed
-
- Kamar N, Selves J, Mansuy J-M, Ouezzani L, Péron J-M, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. - PubMed
-
- Dalton HR, Kamar N, Baylis SA, Moradpour D, Wedemeyer H, Negro F. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–1271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

