Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies
- PMID: 37938100
- PMCID: PMC10635602
- DOI: 10.1097/HC9.0000000000000317
Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies
Abstract
Background: Alpha-fetoprotein (AFP) and des-gamma carboxyprothrombin (DCP), also known as protein induced by vitamin K absence-II (PIVKA-II [DCP]) are biomarkers for HCC with limited diagnostic value when used in isolation. The novel GAAD algorithm is an in vitro diagnostic combining PIVKA-II (DCP) and AFP measurements, age, and gender (biological sex) to generate a semi-quantitative result. We conducted prospective studies to develop, implement, and clinically validate the GAAD algorithm for differentiating HCC (early and all-stage) and benign chronic liver disease (CLD), across disease stages and etiologies.
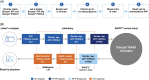
Methods: Patients aged ≥18 years with HCC or CLD were prospectively enrolled internationally into algorithm development [n = 1084; 309 HCC cases (40.7% early-stage) and 736 controls] and clinical validation studies [n = 877; 366 HCC cases (47.6% early-stage) and 303 controls]. Serum samples were analyzed on a cobas® e 601 analyzer. Performance was assessed using receiver operating characteristic curve analyses to calculate AUC.
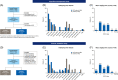
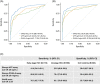
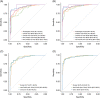
Results: For algorithm development, AUC for differentiation between early-stage HCC and CLD was 90.7%, 84.4%, and 77.2% for GAAD, AFP, and PIVKA-II, respectively. The sensitivity of GAAD for the detection of early-stage HCC was 71.8% with 90.0% specificity. Similar results were shown in the clinical validation study; AUC for differentiation between early-stage HCC and CLD was 91.4% with 70.1% sensitivity and 93.7% specificity. GAAD also showed strong specificity, with a lower rate of false positives regardless of disease stage, etiology, or region.
Conclusions: The GAAD algorithm significantly improves early-stage HCC detection for patients with CLD undergoing HCC surveillance. Further phase III and IV studies are warranted to assess the utility of incorporating the algorithm into clinical practice.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Teerha Piratvisuth is on the speakers’ bureau and received grants from Gilead. He is on the speakers’ bureau for Bristol Myers Squibb, Bayer, Abbott, Eisai, and MSD. He received grants from Roche, Jannsen FibroGen, and VIR. Jinlin Hou advises, is on the speakers’ bureau, and received grants from Gilead. He advises and is on the speakers’ bureau for GlaxoSmithKline and Roche. He advises Aligos, Assembly, and Johnson Pharmaceutica. He received grants from Bristol Myers Squibb. Tawesak Tanwandee received grants from Gilead, Roche, Vir, Merck, and Altimune. Thomas Berg consults for Bayer, Eisai, Ipsen, Merck, and Roche. Arndt Vogel consults, is on the speakers’ bureau, and eceived grants from Sevier and Incyte. He consults and is on the speakers’ bureau for AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, Bristol Myers Squibb, BTG, Daichi-Sankyo, Eisai, Incyte, Ipsen, MSD, PierreFabre, Roche, Servier, Sirtex, Tahio, and Terumo. He is on the speakers’ bureau for GlaxoSmithKline, Imaging Equipment, and Jiangsu Hengrui Medicines. He has other interests with for Onclive and Oncowissen.de. Jörg Trojan consults for Amgen, Bayer Healthcare, Bristol Myers Squibb, Eisai, Ipsen, Merck Serono, Merck, Eli Lilly, ImClone, and Roche. Enrico N De Toni consults, is on the speakers’ bureau, and received grants from Bristol Myers Squibb. He consults and received grants from AstraZeneca, Bayer, Eli Lilly, and Roche. He consults for Eisai, Ipsen, and Pfizer. He is on the speakers’ bureau for Falk. He received grants from ArQule and Celsion. Masatoshi Kudo advises, is on the speakers’ bureau, and received grants Eisai. He advises and is on the speakers’ bureau for Bristol Myers Squibb. He advises and received grants from Ono. He is on the speakers’ bureau and received grants from EA Pharma. He advises MSD and Roche. He is on the speakers’ bureau for Bayer, Eli Lilly, and Merck. He received grants from AbbVie, Gilead, Ono, Otsuka, Sumitomo Dainippon Pharma, Taiho, and Takeda. Anja Eiblmaier is employed by Roche. Kairat Madin is employed by Roche. Konstantin Kroeniger is employed by Roche. Ashish Sharma is employed by Roche. Henry LY Chan consults and is on the speakers’ bureau for Gilead and Roche. He consults for Arbutus, GlaxoSmithKline, Janssen, Vir, Aligos, Vaccitech, and Virion. He is on the speaker’s bureau for Viatris. The remaining authors have no conflicts to report.
Figures



References
-
- International Agency for Research on Cancer. https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf Globocan 2020 Liver fact sheet. Accessed November 2022. 2020;
-
- Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. . Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541–565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

