Xylan utilisation promotes adaptation of Bifidobacterium pseudocatenulatum to the human gastrointestinal tract
- PMID: 37938239
- PMCID: PMC9723692
- DOI: 10.1038/s43705-021-00066-4
Xylan utilisation promotes adaptation of Bifidobacterium pseudocatenulatum to the human gastrointestinal tract
Abstract
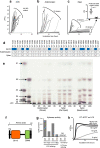
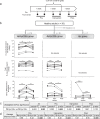
Dietary carbohydrates impact the composition of the human gut microbiota. However, the relationship between carbohydrate availability for individual bacteria and their growth in the intestinal environment remains unclear. Here, we show that the availability of long-chain xylans (LCX), one of the most abundant dietary fibres in the human diet, promotes the growth of Bifidobacterium pseudocatenulatum in the adult human gut. Genomic and phenotypic analyses revealed that the availability of LCX-derived oligosaccharides is a fundamental feature of B. pseudocatenulatum, and that some but not all strains possessing the endo-1,4-β-xylanase (BpXyn10A) gene grow on LCX by cleaving the xylose backbone. The BpXyn10A gene, likely acquired by horizontal transfer, was incorporated into the gene cluster for LCX-derived oligosaccharide utilisation. Co-culturing with xylanolytic Bacteroides spp. demonstrated that LCX-utilising strains are more competitive than LCX non-utilising strains even when LCX-derived oligosaccharides were supplied. In LCX-rich dietary interventions in adult humans, levels of endogenous B. pseudocatenulatum increased only when BpXyn10A was detected, indicating that LCX availability is a fitness determinant in the human gut. Our findings highlight the enhanced intestinal adaptability of bifidobacteria via polysaccharide utilisation, and provide a cornerstone for systematic manipulation of the intestinal microbiota through dietary intervention using key enzymes that degrade polysaccharide as biomarkers.
© 2021. The Author(s).
Conflict of interest statement
This work was funded by the Yakult Central Institute. The funder provided support in the form of salaries for all authors, but did not have any additional role in the study design, data collection and analysis or preparation of the manuscript. Yakult Central Institute is the owner of a patent application based on some of the results presented, which names YW, YS, TH, YA-S and TM as inventors.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

