Benthic exometabolites and their ecological significance on threatened Caribbean coral reefs
- PMID: 37938276
- PMCID: PMC9723752
- DOI: 10.1038/s43705-022-00184-7
Benthic exometabolites and their ecological significance on threatened Caribbean coral reefs
Abstract
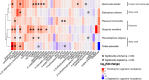
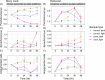
Benthic organisms are the architectural framework supporting coral reef ecosystems, but their community composition has recently shifted on many reefs. Little is known about the metabolites released from these benthic organisms and how compositional shifts may influence other reef life, including prolific microorganisms. To investigate the metabolite composition of benthic exudates and their ecological significance for reef microbial communities, we harvested exudates from six species of Caribbean benthic organisms including stony corals, octocorals, and an invasive encrusting alga, and subjected these exudates to untargeted and targeted metabolomics approaches using liquid chromatography-mass spectrometry. Incubations with reef seawater microorganisms were conducted to monitor changes in microbial abundances and community composition using 16 S rRNA gene sequencing in relation to exudate source and three specific metabolites. Exudates were enriched in amino acids, nucleosides, vitamins, and indole-based metabolites, showing that benthic organisms contribute labile organic matter to reefs. Furthermore, exudate compositions were species-specific, and riboflavin and pantothenic acid emerged as significant coral-produced metabolites, while caffeine emerged as a significant invasive algal-produced metabolite. Microbial abundances and individual microbial taxa responded differently to exudates from stony corals and octocorals, demonstrating that exudate mixtures released from different coral species select for specific bacteria. In contrast, microbial communities did not respond to individual additions of riboflavin, pantothenic acid, or caffeine. This work indicates that recent shifts in benthic organisms alter exudate composition and likely impact microbial communities on coral reefs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Barott KL, Rodriguez-Brito B, Janouškovec J, Marhaver KL, Smith JE, Keeling P, et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol. 2011;13:1192–204. doi: 10.1111/j.1462-2920.2010.02419.x. - DOI - PubMed

