Carbon-infiltrated carbon nanotubes inhibit the development of Staphylococcus aureus biofilms
- PMID: 37938619
- PMCID: PMC10632507
- DOI: 10.1038/s41598-023-46748-y
Carbon-infiltrated carbon nanotubes inhibit the development of Staphylococcus aureus biofilms
Abstract
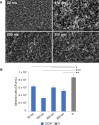
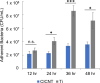
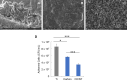
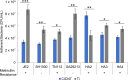
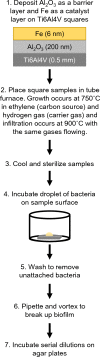
Staphylococcus aureus forms biofilms that cause considerable morbidity and mortality in patients who receive implanted devices such as prosthetics or fixator pins. An ideal surface for such medical devices would inhibit biofilm growth. Recently, it was reported that surface modification of stainless steel materials with carbon-infiltrated carbon nanotubes (CICNT) inhibits the growth of S. aureus biofilms. The purpose of this study was to investigate this antimicrobial effect on titanium materials with CICNT coated surfaces in a variety of surface morphologies and across a broader spectrum of S. aureus isolates. Study samples of CICNT-coated titanium, and control samples of bare titanium, a common implant material, were exposed to S. aureus. Viable bacteria were removed from adhered biofilms and quantified as colony forming units. Scanning electron microscopy was used to qualitatively analyze biofilms both before and after removal of cells. The CICNT surface was found to have significantly fewer adherent bacteria than bare titanium control surfaces, both via colony forming unit and microscopic analyses. This effect was most pronounced on CICNT surfaces with an average nanotube diameter of 150 nm, showing a 2.5-fold reduction in adherent bacteria. Since S. aureus forms different biofilm structures by isolate and by growth conditions, we tested 7 total isolates and found a significant reduction in the biofilm load in six out of seven S. aureus isolates tested. To examine whether the anti-biofilm effect was due to the structure of the nanotubes, we generated an unstructured carbon surface. Significantly more bacteria adhered to a nonstructured carbon surface than to the 150 nm CICNT surface, suggesting that the topography of the nanotube structure itself has anti-biofilm properties. The CICNT surface possesses anti-biofilm properties that result in fewer adherent S. aureus bacteria. These anti-biofilm properties are consistent across multiple isolates of S. aureus and are affected by nanotube diameter. The experiments performed in this study suggest that this effect is due to the nanostructure of the CICNT surface.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- 2018 Hospital Inpatient National Statistics. https://hcupnet.ahrq.gov/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

