The plant rhizosheath-root niche is an edaphic "mini-oasis" in hyperarid deserts with enhanced microbial competition
- PMID: 37938683
- PMCID: PMC9723607
- DOI: 10.1038/s43705-022-00130-7
The plant rhizosheath-root niche is an edaphic "mini-oasis" in hyperarid deserts with enhanced microbial competition
Abstract
Plants have evolved unique morphological and developmental adaptations to cope with the abiotic stresses imposed by (hyper)arid environments. Such adaptations include the formation of rhizosheath-root system in which mutualistic plant-soil microbiome associations are established: the plant provides a nutrient-rich and shielded environment to microorganisms, which in return improve plant-fitness through plant growth promoting services. We hypothesized that the rhizosheath-root systems represent refuge niches and resource islands for the desert edaphic microbial communities. As a corollary, we posited that microorganisms compete intensively to colonize such "oasis" and only those beneficial microorganisms improving host fitness are preferentially selected by plant. Our results show that the belowground rhizosheath-root micro-environment is largely more hospitable than the surrounding gravel plain soil with higher nutrient and humidity contents, and cooler temperatures. By combining metabarcoding and shotgun metagenomics, we demonstrated that edaphic microbial biomass and community stability increased from the non-vegetated soils to the rhizosheath-root system. Concomitantly, non-vegetated soil communities favored autotrophy lifestyle while those associated with the plant niches were mainly heterotrophs and enriched in microbial plant growth promoting capacities. An intense inter-taxon microbial competition is involved in the colonization and homeostasis of the rhizosheath zone, as documented by significant enrichment of antibiotic resistance genes and CRISPR-Cas motifs. Altogether, our results demonstrate that rhizosheath-root systems are "edaphic mini-oases" and microbial diversity hotspots in hyperarid deserts. However, to colonize such refuge niches, the desert soil microorganisms compete intensively and are therefore prepared to outcompete potential rivals.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


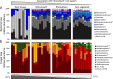



References
-
- Laity JJ. Deserts and desert environments. John Wiley & Sons; UK, 2009.
-
- Huang J, Yu H, Guan X, Wang G, Guo R. Accelerated dryland expansion under climate change. Nat Clim Chang. 2015;6:166–71. doi: 10.1038/nclimate2837. - DOI
-
- Danin A. Plant adaptations to environmental stresses in desert dunes. In: Cloudsley-Thompson J, Punzo F, editors. Adaptations of desert organisms. Plant of desert dunes. Springer; Verlag Berlin Heidelberg, 1996.
LinkOut - more resources
Full Text Sources
Research Materials

