Scrambling the genome in cancer: causes and consequences of complex chromosome rearrangements
- PMID: 37938738
- PMCID: PMC10922386
- DOI: 10.1038/s41576-023-00663-0
Scrambling the genome in cancer: causes and consequences of complex chromosome rearrangements
Abstract
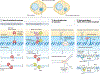
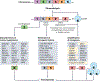
Complex chromosome rearrangements, known as chromoanagenesis, are widespread in cancer. Based on large-scale DNA sequencing of human tumours, the most frequent type of complex chromosome rearrangement is chromothripsis, a massive, localized and clustered rearrangement of one (or a few) chromosomes seemingly acquired in a single event. Chromothripsis can be initiated by mitotic errors that produce a micronucleus encapsulating a single chromosome or chromosomal fragment. Rupture of the unstable micronuclear envelope exposes its chromatin to cytosolic nucleases and induces chromothriptic shattering. Found in up to half of tumours included in pan-cancer genomic analyses, chromothriptic rearrangements can contribute to tumorigenesis through inactivation of tumour suppressor genes, activation of proto-oncogenes, or gene amplification through the production of self-propagating extrachromosomal circular DNAs encoding oncogenes or genes conferring anticancer drug resistance. Here, we discuss what has been learned about the mechanisms that enable these complex genomic rearrangements and their consequences in cancer.
© 2023. Springer Nature Limited.
Figures



References
-
- Nowell PC & Hungerford DA Minute Chromosome in Human Chronic Granulocytic Leukemia. Science 132, 1497–1497 (1960).
-
- Ishihara T, Kikuchi Y. & Sandberg AA Chromosomes of twenty cancer effusions: correlation of karyotypic, clinical, and pathologic aspects. J Natl Cancer Inst 30, 1303–1361 (1963). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

