Single-molecule dynamics show a transient lipopolysaccharide transport bridge
- PMID: 37938784
- PMCID: PMC10842706
- DOI: 10.1038/s41586-023-06709-x
Single-molecule dynamics show a transient lipopolysaccharide transport bridge
Abstract
Gram-negative bacteria are surrounded by two membranes. A special feature of the outer membrane is its asymmetry. It contains lipopolysaccharide (LPS) in the outer leaflet and phospholipids in the inner leaflet1-3. The proper assembly of LPS in the outer membrane is required for cell viability and provides Gram-negative bacteria intrinsic resistance to many classes of antibiotics. LPS biosynthesis is completed in the inner membrane, so the LPS must be extracted, moved across the aqueous periplasm that separates the two membranes and translocated through the outer membrane where it assembles on the cell surface4. LPS transport and assembly requires seven conserved and essential LPS transport components5 (LptA-G). This system has been proposed to form a continuous protein bridge that provides a path for LPS to reach the cell surface6,7, but this model has not been validated in living cells. Here, using single-molecule tracking, we show that Lpt protein dynamics are consistent with the bridge model. Half of the inner membrane Lpt proteins exist in a bridge state, and bridges persist for 5-10 s, showing that their organization is highly dynamic. LPS facilitates Lpt bridge formation, suggesting a mechanism by which the production of LPS can be directly coupled to its transport. Finally, the bridge decay kinetics suggest that there may be two different types of bridges, whose stability differs according to the presence (long-lived) or absence (short-lived) of LPS. Together, our data support a model in which LPS is both a substrate and a structural component of dynamic Lpt bridges that promote outer membrane assembly.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest declaration
The authors declare no competing interests.
Figures
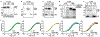

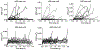




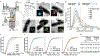

References
-
- Osborn M, Gander J, Parisi E & Carson J Mechanism of assembly of the outer membrane of Salmonella typhimurium: isolation and characterization of cytoplasmic and outer membrane. Journal of Biological Chemistry 247, 3962–3972 (1972). - PubMed
-
- Kamio Y & Nikaido H Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 15, 2561–2570 (1976). - PubMed
-
- Mühlradt PF & Golecki JR Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. European journal of biochemistry 51, 343–352 (1975). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

