Assessing Squarates as Amine-Reactive Probes
- PMID: 37938802
- PMCID: PMC10935565
- DOI: 10.1021/jacs.2c05691
Assessing Squarates as Amine-Reactive Probes
Abstract
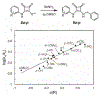
Probes that covalently label protein targets facilitate the identification of ligand-binding sites. Lysine residues are prevalent in the proteome, making them attractive substrates for covalent probes. However, identifying electrophiles that undergo amine-specific, regioselective reactions with binding site lysine residues is challenging. Squarates can engage in two sequential conjugate addition-elimination reactions with amines. Nitrogen donation reduces the second reaction rate, making the mono squaramide a mild electrophile. We postulated that this mild electrophilicity would demand a longer residence time near the amine, affording higher selectivity for binding site lysines. Therefore, we compared the kinetics of squarate and monosquaramide amine substitution to alternative amine bioconjugation handles. The data revealed that N-hydroxy succinimidyl esters react 4 orders of magnitude faster, consistent with their labeling promiscuity. Squarate reactivity can be tuned by a substitution pattern. Electron-withdrawing groups on the vinylogous ester or amide increase reaction rates. Dithionosquarates react more rapidly than squarates, while vinylogous thioester analogs, dithiosquarates, react more slowly. We assessed squarate selectively using the UDP-sugar processing enzyme GlfT2 from Mycobacterium tuberculosis, which possesses 21 surface-exposed lysines. The reaction predominately modified one lysine proximal to a binding site to afford covalent inhibition. These findings demonstrate the selectivity of squaric esters and squaramides, which is a critical feature for affinity-based chemoproteomic probes.
Figures
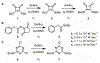

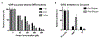
References
-
- Hu Q-YY; Allan M; Adamo R; Quinn D; Zhai H; Wu G; Clark K; Zhou J; Ortiz S; Wang B; et al. Synthesis of a Well-Defined Glycoconjugate Vaccine by a Tyrosine-Selective Conjugation Strategy. Chem. Sci 2013, 4 (10), 3827–3832.
-
- Kallemeijn WW; Li K-YY; Witte MD; Marques ARAA; Aten J; Scheij S; Jiang J; Willems LI; Voorn-Brouwer TM; van Roomen CPAA; et al. Novel Activity-Based Probes for Broad-Spectrum Profiling of Retaining β-Exoglucosidases in Situ and in Vivo. Angew. Chemie - Int. Ed 2012, 51, 12529–12533. 10.1002/anie.201207771. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

