Data-driven epidemiologic approach to conducting site feasibility for a global phase III tuberculosis vaccine clinical trial
- PMID: 37939024
- PMCID: PMC10631637
- DOI: 10.1371/journal.pgph.0002544
Data-driven epidemiologic approach to conducting site feasibility for a global phase III tuberculosis vaccine clinical trial
Abstract
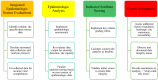
An efficacious tuberculosis (TB) vaccine is critical to reducing the global burden of TB. TB vaccine trials require the identification of multiple sites globally that have both a high incidence of TB and the capacity to conduct a clinical trial. To expand the diversity of potential phase III TB vaccine trial sites to be considered for inclusion, we describe a novel epidemiologic method that incorporates approaches from a variety of public health practices. Our approach incorporates analytic methodology to enable quantification and validation of qualitative information from disparate data sources, and epidemiologic analysis to systematically assess site-specific TB epidemiology. The integration of robust data-driven practices, and more quantitatively focused analysis, allowed for the objective evaluation of sites, which resulted in the identification of sites and catchment areas with high TB burden that may not have been previously considered. This suggests that an integrated epidemiologic methodology, not traditionally utilized for clinical trial site evaluations, could be integrated into site feasibility assessments as it results in more rapid site identification and reduces unintended bias.
Copyright: © 2023 Mui et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist. ACS, AFD, CP are employees of the Bill & Melinda Gates Medical Research Institute, a non-profit organization dedicated to the development and effective use of novel biomedical interventions aimed at reducing the burden of TB, malaria, diarrheal diseases, and maternal, newborn, and child illnesses worldwide.
Figures

References
-
- World Health Organization. Global tuberculosis report 2020. 2020. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
-
- Frick M. Pipeline Report 2020: Tuberculosis Vaccines. 2020.
LinkOut - more resources
Full Text Sources
