Immune activation state modulates infant engram expression across development
- PMID: 37939176
- PMCID: PMC10631722
- DOI: 10.1126/sciadv.adg9921
Immune activation state modulates infant engram expression across development
Abstract
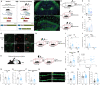
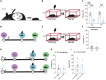
Infantile amnesia is possibly the most ubiquitous form of memory loss in mammals. We investigated how memories are stored in the brain throughout development by integrating engram labeling technology with mouse models of infantile amnesia. Here, we found a phenomenon in which male offspring in maternal immune activation models of autism spectrum disorder do not experience infantile amnesia. Maternal immune activation altered engram ensemble size and dendritic spine plasticity. We rescued the same apparently forgotten infantile memories in neurotypical mice by optogenetically reactivating dentate gyrus engram cells labeled during complex experiences in infancy. Furthermore, we permanently reinstated lost infantile memories by artificially updating the memory engram, demonstrating that infantile amnesia is a reversible process. Our findings suggest not only that infantile amnesia is due to a reversible retrieval deficit in engram expression but also that immune activation during development modulates innate, and reversible, forgetting switches that determine whether infantile amnesia will occur.
Figures



References
-
- C. Rovee-Collier, The development of infant memory. Curr. Dir. Psychol. Sci. 8, 80–85 (1999).
-
- N. S. Newcombe, A. B. Drummey, N. A. Fox, E. Lie, W. Ottinger-Alberts, Remembering early childhood: How much, how, and why (or why not). Curr. Dir. Psychol. Sci. 9, 55–58 (2000).
-
- B. A. Campbell, E. H. Campbell, Retention and extinction of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 55, 1–8 (1962). - PubMed
-
- K. G. Akers, A. Martinez-Canabal, L. Restivo, A. P. Yiu, A. De Cristofaro, H.-L. L. Hsiang, A. L. Wheeler, A. Guskjolen, Y. Niibori, H. Shoji, K. Ohira, B. A. Richards, T. Miyakawa, S. A. Josselyn, P. W. Frankland, Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

