Truly pattern: Nonlinear integration of motion signals is required to account for the responses of pattern cells in rat visual cortex
- PMID: 37939191
- PMCID: PMC10631736
- DOI: 10.1126/sciadv.adh4690
Truly pattern: Nonlinear integration of motion signals is required to account for the responses of pattern cells in rat visual cortex
Abstract
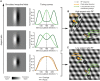
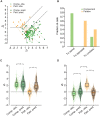
A key feature of advanced motion processing in the primate dorsal stream is the existence of pattern cells-specialized cortical neurons that integrate local motion signals into pattern-invariant representations of global direction. Pattern cells have also been reported in rodent visual cortex, but it is unknown whether the tuning of these neurons results from truly integrative, nonlinear mechanisms or trivially arises from linear receptive fields (RFs) with a peculiar geometry. Here, we show that pattern cells in rat primary (V1) and lateromedial (LM) visual cortex process motion direction in a way that cannot be explained by the linear spatiotemporal structure of their RFs. Instead, their tuning properties are consistent with and well explained by those of units in a state-of-the-art neural network model of the dorsal stream. This suggests that similar cortical processes underlay motion representation in primates and rodents. The latter could thus serve as powerful model systems to unravel the underlying circuit-level mechanisms.
Figures







Similar articles
-
Unique Spatial Integration in Mouse Primary Visual Cortex and Higher Visual Areas.J Neurosci. 2020 Feb 26;40(9):1862-1873. doi: 10.1523/JNEUROSCI.1997-19.2020. Epub 2020 Jan 16. J Neurosci. 2020. PMID: 31949109 Free PMC article.
-
Nonlinear Processing of Shape Information in Rat Lateral Extrastriate Cortex.J Neurosci. 2019 Feb 27;39(9):1649-1670. doi: 10.1523/JNEUROSCI.1938-18.2018. Epub 2019 Jan 7. J Neurosci. 2019. PMID: 30617210 Free PMC article.
-
Pattern and Component Motion Responses in Mouse Visual Cortical Areas.Curr Biol. 2015 Jun 29;25(13):1759-64. doi: 10.1016/j.cub.2015.05.028. Epub 2015 Jun 11. Curr Biol. 2015. PMID: 26073133 Free PMC article.
-
Nonlinear Y-Like Receptive Fields in the Early Visual Cortex: An Intermediate Stage for Building Cue-Invariant Receptive Fields from Subcortical Y Cells.J Neurosci. 2017 Jan 25;37(4):998-1013. doi: 10.1523/JNEUROSCI.2120-16.2016. J Neurosci. 2017. PMID: 28123031 Free PMC article.
-
Auditory and Visual Motion Processing and Integration in the Primate Cerebral Cortex.Front Neural Circuits. 2018 Oct 26;12:93. doi: 10.3389/fncir.2018.00093. eCollection 2018. Front Neural Circuits. 2018. PMID: 30416431 Free PMC article. Review.
Cited by
-
Cortical circuits for cross-modal generalization.Nat Commun. 2025 May 26;16(1):4230. doi: 10.1038/s41467-025-59342-9. Nat Commun. 2025. PMID: 40419471 Free PMC article.
References
-
- D. Marr, Vision: A Computational Investigation into the Human Representation and Processing of Visual Information (MIT Press, 2010).
-
- C. L. Fennema, W. B. Thompson, Velocity determination in scenes containing several moving objects. Comput. Graph. Image Process. 9, 301–315 (1979).
-
- S. Wüerger, R. Shapley, N. Rubin, “On the visually perceived direction of motion” by Hans Wallach: 60 years later. Perception 25, 1317–1367 (1996).
-
- J. A. Movshon, E. H. Adelson, M. S. Gizzi, W. T. Newsome, C. Chagas, R. Gattass, C. Gross, Pattern Recognition Mechanisms (Springer-Verlag, 1985).
-
- H. R. Rodman, T. D. Albright, Single-unit analysis of pattern-motion selective properties in the middle temporal visual area (MT). Exp. Brain Res. 75, 53–62 (1989). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

