Inhibition of the renal apical sodium dependent bile acid transporter prevents cholemic nephropathy in mice with obstructive cholestasis
- PMID: 37939855
- PMCID: PMC10849134
- DOI: 10.1016/j.jhep.2023.10.035
Inhibition of the renal apical sodium dependent bile acid transporter prevents cholemic nephropathy in mice with obstructive cholestasis
Erratum in
-
Erratum to: 'Inhibition of the renal apical sodium dependent bile acid transporter prevents cholemic nephropathy in mice with obstructive cholestasis' [J Hepatol (2024) 80:268-281].J Hepatol. 2025 Jun;82(6):1140. doi: 10.1016/j.jhep.2025.02.031. Epub 2025 Mar 15. J Hepatol. 2025. PMID: 40090770 Free PMC article. No abstract available.
Abstract
Background & aims: Cholemic nephropathy (CN) is a severe complication of cholestatic liver diseases for which there is no specific treatment. We revisited its pathophysiology with the aim of identifying novel therapeutic strategies.
Methods: Cholestasis was induced by bile duct ligation (BDL) in mice. Bile flux in kidneys and livers was visualized by intravital imaging, supported by MALDI mass spectrometry imaging and liquid chromatography-tandem mass spectrometry. The effect of AS0369, a systemically bioavailable apical sodium-dependent bile acid transporter (ASBT) inhibitor, was evaluated by intravital imaging, RNA-sequencing, histological, blood, and urine analyses. Translational relevance was assessed in kidney biopsies from patients with CN, mice with a humanized bile acid (BA) spectrum, and via analysis of serum BAs and KIM-1 (kidney injury molecule 1) in patients with liver disease and hyperbilirubinemia.
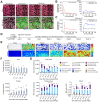
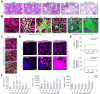
Results: Proximal tubular epithelial cells (TECs) reabsorbed and enriched BAs, leading to oxidative stress and death of proximal TECs, casts in distal tubules and collecting ducts, peritubular capillary leakiness, and glomerular cysts. Renal ASBT inhibition by AS0369 blocked BA uptake into TECs and prevented kidney injury up to 6 weeks after BDL. Similar results were obtained in mice with humanized BA composition. In patients with advanced liver disease, serum BAs were the main determinant of KIM-1 levels. ASBT expression in TECs was preserved in biopsies from patients with CN, further highlighting the translational potential of targeting ASBT to treat CN.
Conclusions: BA enrichment in proximal TECs followed by oxidative stress and cell death is a key early event in CN. Inhibiting renal ASBT and consequently BA enrichment in TECs prevents CN and systemically decreases BA concentrations.
Impact and implications: Cholemic nephropathy (CN) is a severe complication of cholestasis and an unmet clinical need. We demonstrate that CN is triggered by the renal accumulation of bile acids (BAs) that are considerably increased in the systemic blood. Specifically, the proximal tubular epithelial cells of the kidney take up BAs via the apical sodium-dependent bile acid transporter (ASBT). We developed a therapeutic compound that blocks ASBT in the kidneys, prevents BA overload in tubular epithelial cells, and almost completely abolished all disease hallmarks in a CN mouse model. Renal ASBT inhibition represents a potential therapeutic strategy for patients with CN.
Keywords: Cholestasis; bile cast nephropathy; bile duct ligation; intravital imaging; kidney injury.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures







Comment in
-
Detour of bile acid routes as therapeutic roadmap for cholemic nephropathy.J Hepatol. 2024 Feb;80(2):188-190. doi: 10.1016/j.jhep.2023.11.008. Epub 2023 Nov 25. J Hepatol. 2024. PMID: 38013144 No abstract available.
References
-
- EASL. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. - PubMed
-
- Krones E., Pollheimer M.J., Rosenkranz A.R., et al. Cholemic nephropathy - historical notes and novel perspectives. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1356–1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

