Platelet biology and function: plaque erosion vs. rupture
- PMID: 37940193
- PMCID: PMC10757869
- DOI: 10.1093/eurheartj/ehad720
Platelet biology and function: plaque erosion vs. rupture
Abstract
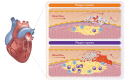
The leading cause of heart disease in developed countries is coronary atherosclerosis, which is not simply a result of ageing but a chronic inflammatory process that can lead to acute clinical events upon atherosclerotic plaque rupture or erosion and arterial thrombus formation. The composition and location of atherosclerotic plaques determine the phenotype of the lesion and whether it is more likely to rupture or to erode. Although plaque rupture and erosion both initiate platelet activation on the exposed vascular surface, the contribution of platelets to thrombus formation differs between the two phenotypes. In this review, plaque phenotype is discussed in relation to thrombus composition, and an overview of important mediators (haemodynamics, matrix components, and soluble factors) in plaque-induced platelet activation is given. As thrombus formation on disrupted plaques does not necessarily result in complete vessel occlusion, plaque healing can occur. Therefore, the latest findings on plaque healing and the potential role of platelets in this process are summarized. Finally, the clinical need for more effective antithrombotic agents is highlighted.
Keywords: Plaque erosion; Plaque rupture; Platelet activation; Thrombus formation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

