Heterogeneity of determining disease severity, clinical course and outcomes in systemic sclerosis-associated interstitial lung disease: a systematic literature review
- PMID: 37940340
- PMCID: PMC10632935
- DOI: 10.1136/rmdopen-2023-003426
Heterogeneity of determining disease severity, clinical course and outcomes in systemic sclerosis-associated interstitial lung disease: a systematic literature review
Abstract
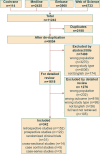
Objective The course of systemic sclerosis-associated interstitial lung disease (SSc-ILD) is highly variable and different from continuously progressive idiopathic pulmonary fibrosis (IPF). Most proposed definitions of progressive pulmonary fibrosis or SSc-ILD severity are based on the research data from patients with IPF and are not validated for patients with SSc-ILD. Our study aimed to gather the current evidence for severity, progression and outcomes of SSc-ILD.Methods A systematic literature review to search for definitions of severity, progression and outcomes recorded for SSc-ILD was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines in Medline, Embase, Web of Science and Cochrane Library up to 1 August 2023.Results A total of 9054 papers were reviewed and 342 were finally included. The most frequent tools used for the definition of SSc-ILD progression and severity were combined changes of carbon monoxide diffusing capacity (DLCO) and forced vital capacity (FVC), isolated FVC or DLCO changes, high-resolution CT (HRCT) extension and composite algorithms including pulmonary function test, clinical signs and HRCT data. Mortality was the most frequently reported long-term event, both from all causes or ILD related.Conclusions The studies presenting definitions of SSc-ILD 'progression', 'severity' and 'outcome' show a large heterogeneity. These results emphasise the need for developing a standardised, consensus definition of severe SSc-ILD, to link a disease specific definition of progression as a surrogate outcome for clinical trials and clinical practice.PROSPERO registration number CRD42022379254.Cite Now.
Keywords: Epidemiology; Outcome Assessment, Health Care; Pulmonary Fibrosis; Systemic Sclerosis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Liubov Petelytska Grant/research support from: received research grant from Swiss National Research Foundation/Scholars at risk. Francesco Bonomi, Carlo Cannistrà, Elisa Fiorentini, Silvia Peretti, Sara Torracchi, Pamela Bernardini, Carmela Coccia, Riccardo De Luca, Alessio Economou, Juela Levani: None declared. Marco Matucci-Cerinic: Speakers bureau: Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, Roche., Consultant of: Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, Roche. Distler O: Consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Prometheus, Redxpharma, Roivant and Topadur. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Co-founder of CITUS AG. Editorial Board member for Annals of the Rheumatic Diseases, RMD Open. Cosimo Bruni Speakers bureau: Eli- Lilly, Consultant of: Boehringer Ingelheim, Grant/research support from: Gruppo Italiano Lotta alla Sclerodermia (GILS), European Scleroderma Trials and Research Group (EUSTAR), Foundation for research in Rheumatology (FOREUM), Scleroderma Clinical Trials Consortium (SCTC), Scleroderma Research Foundation (SRF). Educational grants from AbbVie and Wellcome Trust. Congress Support from: Boehringer Ingelheim.
Figures
References
-
- Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. Journal of Scleroderma and Related Disorders 2017;2:137–52. 10.5301/jsrd.5000249 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical