Advances in Deep Brain Stimulation: From Mechanisms to Applications
- PMID: 37940596
- PMCID: PMC10634582
- DOI: 10.1523/JNEUROSCI.1427-23.2023
Advances in Deep Brain Stimulation: From Mechanisms to Applications
Abstract
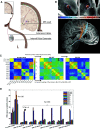
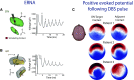
Deep brain stimulation (DBS) is an effective therapy for various neurologic and neuropsychiatric disorders, involving chronic implantation of electrodes into target brain regions for electrical stimulation delivery. Despite its safety and efficacy, DBS remains an underutilized therapy. Advances in the field of DBS, including in technology, mechanistic understanding, and applications have the potential to expand access and use of DBS, while also improving clinical outcomes. Developments in DBS technology, such as MRI compatibility and bidirectional DBS systems capable of sensing neural activity while providing therapeutic stimulation, have enabled advances in our understanding of DBS mechanisms and its application. In this review, we summarize recent work exploring DBS modulation of target networks. We also cover current work focusing on improved programming and the development of novel stimulation paradigms that go beyond current standards of DBS, many of which are enabled by sensing-enabled DBS systems and have the potential to expand access to DBS.
Keywords: coordinated reset; deep brain stimulation; evoked potentials; functional magnetic resonance imaging; sweet spot mapping.
Copyright © 2023 the authors.
Figures

References
-
- Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, Silchenko A, Volkmann J, Deuschl G, Meissner WG, Maarouf M, Sturm V, Freund HJ, Tass PA (2014) Coordinated reset neuromodulation for Parkinson's disease: proof-of-concept study. Mov Disord 29:1679–1684. 10.1002/mds.25923 - DOI - PMC - PubMed
-
- Aman JE, Johnson LA, Sanabria DE, Wang J, Patriat R, Hill M, Marshall E, MacKinnon CD, Cooper SE, Schrock LE, Park MC, Harel N, Vitek JL (2020) Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson's disease patients. Neurobiol Dis 139:104819. 10.1016/j.nbd.2020.104819 - DOI - PMC - PubMed
-
- Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, Cogiamanian F, Prenassi M, Bocci T, Cortese F, Rampini P, Barbieri S, Priori A (2018) Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90:e971–e976. 10.1212/WNL.0000000000005121 - DOI - PMC - PubMed
