2022 Peritoneal Surface Oncology Group International Consensus on HIPEC Regimens for Peritoneal Malignancies: Colorectal Cancer
- PMID: 37940803
- PMCID: PMC10695877
- DOI: 10.1245/s10434-023-14368-5
2022 Peritoneal Surface Oncology Group International Consensus on HIPEC Regimens for Peritoneal Malignancies: Colorectal Cancer
Abstract
Background: Selected patients with peritoneal metastases of colorectal cancer (PM-CRC) can benefit from potentially curative cytoreductive surgery (CRS) ± hyperthermic intraperitoneal chemotherapy (HIPEC), with a median overall survival (OS) of more than 40 months.
Objective: The aims of this evidence-based consensus were to define the indications for HIPEC, to select the preferred HIPEC regimens, and to define research priorities regarding the use of HIPEC for PM-CRC.
Methods: The consensus steering committee elaborated and formulated pertinent clinical questions according to the PICO (patient, intervention, comparator, outcome) method and assessed the evidence according to the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) framework. Standardized evidence tables were presented to an international expert panel to reach a consensus (4-point, weak and strong positive/negative) on HIPEC regimens and research priorities through a two-round Delphi process. The consensus was defined as ≥ 50% agreement for the 4-point consensus grading or ≥ 70% for either of the two combinations.
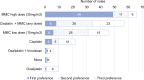
Results: Evidence was weak or very weak for 9/10 clinical questions. In total, 70/90 eligible panelists replied to both Delphi rounds (78%), with a consensus for 10/10 questions on HIPEC regimens. There was strong negative consensus concerning the short duration, high-dose oxaliplatin (OX) protocol (55.7%), and a weak positive vote (53.8-64.3%) in favor of mitomycin-C (MMC)-based HIPEC (preferred choice: Dutch protocol: 35 mg/m2, 90 min, three fractions), both for primary cytoreduction and recurrence. Determining the role of HIPEC after CRS was considered the most important research question, regarded as essential by 85.7% of the panelists. Furthermore, over 90% of experts suggest performing HIPEC after primary and secondary CRS for recurrence > 1 year after the index surgery.
Conclusions: Based on the available evidence, despite the negative results of PRODIGE 7, HIPEC could be conditionally recommended to patients with PM-CRC after CRS. While more preclinical and clinical data are eagerly awaited to harmonize the procedure further, the MMC-based Dutch protocol remains the preferred regimen after primary and secondary CRS.
Keywords: Cytoreductive surgery; Hyperthermic intraperitoneal chemotherapy; Peritoneal metastases; Peritoneal surface malignancies; Treatment regimens.
© 2023. The Author(s).
Figures



References
-
- Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. doi: 10.1016/S1470-2045(16)30500-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

