Can we achieve better trial recruitment by presenting patient information through multimedia? Meta-analysis of 'studies within a trial' (SWATs)
- PMID: 37940944
- PMCID: PMC10634086
- DOI: 10.1186/s12916-023-03081-5
Can we achieve better trial recruitment by presenting patient information through multimedia? Meta-analysis of 'studies within a trial' (SWATs)
Abstract
Background: People need high-quality information to make decisions about research participation. Providing information in written format alone is conventional but may not be the most effective and acceptable approach. We developed a structure for the presentation of information using multimedia which included generic and trial-specific content. Our aim was to embed 'Studies Within A Trial' (SWATs) across multiple ongoing trials to test whether multimedia presentation of patient information led to better rates of recruitment.
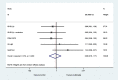
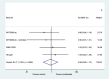
Methods: Five trials included a SWAT and randomised their participants to receive a multimedia presentation alongside standard information, or standard written information alone. We collected data on trial recruitment, acceptance and retention and analysed the pooled results using random effects meta-analysis, with the primary outcome defined as the proportion of participants randomised following an invitation to take part.
Results: Five SWATs provided data on the primary outcome of proportion of participants randomised. Multimedia alongside written information results in little or no difference in recruitment rates (pooled odds ratio = 0.96, 95% CI: 0.79 to 1.17, p-value = 0.671, I2 = 0%). There was no effect on any other outcomes.
Conclusions: Multimedia alongside written information did not improve trial recruitment rates.
Trial registration: ISRCTN71952900, ISRCTN 06710391, ISRCTN 17160087, ISRCTN05926847, ISRCTN62869767.
Keywords: Information; Meta-analysis; Randomised controlled trial; Recruitment; Research methodology; SWATs; User testing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Treweek S, Lockhart P, PitKethly M, Cook J, Kjeldstrøm M, Johansen M, Taskila T, Sullivan F, Wilson S, Jackson C, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3:e002360. doi: 10.1136/bmjopen-2012-002360. - DOI - PMC - PubMed
-
- Rick J, Graffy J, Knapp P, Small N, Collier D, Eldridge S, Kennedy A, Salisbury C, Treweek S, Torgerson D, et al. Systematic Techniques for Assisting Recruitment to Trials (START): study protocol and preliminary findings on a platform for nesting studies of recruitment interventions across multiple trials. Trials. 2014;15:407. doi: 10.1186/1745-6215-15-407. - DOI - PMC - PubMed
-
- Martin-Kerry J, Bower P, Young B, Graffy J, Sheridan R, Watt I, Baines P, Stones C, Preston J, Higgins S, et al. Developing and evaluating multimedia information resources to improve engagement of children, adolescents, and their parents with trials (TRECA study): Study protocol for a series of linked randomised controlled trials. Trials. 2017;18(1):265. doi: 10.1186/s13063-017-1962-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

