Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/MERTK signaling following brain injury
- PMID: 37941008
- PMCID: PMC10633953
- DOI: 10.1186/s12974-023-02940-5
Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/MERTK signaling following brain injury
Abstract
Background: Efferocytosis is a process that removes apoptotic cells and cellular debris. Clearance of these cells alleviates neuroinflammation, prevents the release of inflammatory molecules, and promotes the production of anti-inflammatory cytokines to help maintain tissue homeostasis. The underlying mechanisms by which this occurs in the brain after injury remain ill-defined.
Methods: We used GFP bone marrow chimeric knockout (KO) mice to demonstrate that the axon guidance molecule EphA4 receptor tyrosine kinase is involved in suppressing MERTK in the brain to restrict efferocytosis of resident microglia and peripheral-derived monocyte/macrophages.
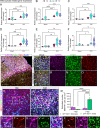
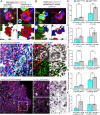
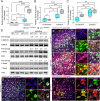
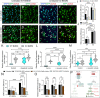
Results: Single-cell RNAseq identified MERTK expression, the primary receptor involved in efferocytosis, on monocytes, microglia, and a subset of astrocytes in the damaged cortex following brain injury. Loss of EphA4 on infiltrating GFP-expressing immune cells improved functional outcome concomitant with enhanced efferocytosis and overall protein expression of p-MERTK, p-ERK, and p-Stat6. The percentage of GFP+ monocyte/macrophages and resident microglia engulfing NeuN+ or TUNEL+ cells was significantly higher in KO chimeric mice. Importantly, mRNA expression of Mertk and its cognate ligand Gas6 was significantly elevated in these mice compared to the wild-type. Analysis of cell-specific expression showed that p-ERK and p-Stat6 co-localized with MERTK-expressing GFP + cells in the peri-lesional area of the cortex following brain injury. Using an in vitro efferocytosis assay, co-culturing pHrodo-labeled apoptotic Jurkat cells and bone marrow (BM)-derived macrophages, we demonstrate that efferocytosis efficiency and mRNA expression of Mertk and Gas6 was enhanced in the absence of EphA4. Selective inhibitors of ERK and Stat6 attenuated this effect, confirming that EphA4 suppresses monocyte/macrophage efferocytosis via inhibition of the ERK/Stat6 pathway.
Conclusions: Our findings implicate the ERK/Stat6/MERTK axis as a novel regulator of apoptotic debris clearance in brain injury that is restricted by peripheral myeloid-derived EphA4 to prevent the resolution of inflammation.
Keywords: Apoptosis; Efferocytosis; Eph; Ephrin; MERTK; Microglia; Neuroinflammation; Peripheral-derived macrophages; Traumatic brain injury.
© 2023. The Author(s).
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


Update of
-
Efferocytosis is restricted by axon guidance molecule EphA4 via ERK/Stat6/Mertk signaling following brain injury.Res Sq [Preprint]. 2023 Jun 27:rs.3.rs-3079466. doi: 10.21203/rs.3.rs-3079466/v1. Res Sq. 2023. Update in: J Neuroinflammation. 2023 Nov 9;20(1):256. doi: 10.1186/s12974-023-02940-5. PMID: 37461720 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

