Analysis of the virulence, infection process, and extracellular enzyme activities of Aspergillus nomius against the Asian corn borer, Ostrinia furnacalis guenée (Lepidoptera: Crambidae)
- PMID: 37941402
- PMCID: PMC10653701
- DOI: 10.1080/21505594.2023.2265108
Analysis of the virulence, infection process, and extracellular enzyme activities of Aspergillus nomius against the Asian corn borer, Ostrinia furnacalis guenée (Lepidoptera: Crambidae)
Abstract
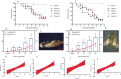
The control of Ostrinia furnacalis, a major pest of maize in Xinjiang, is challenging owing to the occurrence of resistant individuals. Entomopathogenic fungi (EPF) are natural insect regulators used as substitutes for synthetic chemical insecticides. The fungus Aspergillus nomius is highly pathogenic to O. furnacalis; however, its virulence characteristics have not been identified. This study aimed to analyse the lethal efficacy, mode of infection on the cuticle, and extracellular enzyme activity of A. nomius against O. furnacalis. We found that the mortality and mycosis of O. furnacalis were dose-dependent when exposed to A. nomius and varied at different life stages. The egg-hatching and adult emergence rates decreased with an increase in conidial suspension. The highest mortality (83.33%, 7 d post-infection [DPI]) and mycosis (74.33%, 7 DPI) and the lowest mortality response (8.52 × 103 conidia mL-1) and median lethal time (4.91 d) occurred in the 3rd instar larvae of O. furnacalis. Scanning electron microscopy indicated that numerous conidia germination and infection structure formation may have contributed to the high pathogenicity of A. nomius against O. furnacalis. There were significant correlations between O. furnacalis mortality and the activities of extracellular protease, lipase, and chitinase of A. nomius. This study revealed the infection process of the highly pathogenic A. nomius against O. furnacalis, providing a theoretical basis and reference for strain improvement and field application of EPF.
Keywords: Aspergillus nomius; cuticle; extracellular enzymes activity; infection processes; lethal efficacy; ostrinia furnacalis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures










References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous
