Antibody Responses to Influenza Vaccination are Diminished in Patients With Inflammatory Bowel Disease on Infliximab or Tofacitinib
- PMID: 37941436
- PMCID: PMC11037107
- DOI: 10.1093/ecco-jcc/jjad182
Antibody Responses to Influenza Vaccination are Diminished in Patients With Inflammatory Bowel Disease on Infliximab or Tofacitinib
Abstract
Background and aims: We sought to determine whether six commonly used immunosuppressive regimens were associated with lower antibody responses after seasonal influenza vaccination in patients with inflammatory bowel disease [IBD].
Methods: We conducted a prospective study including 213 IBD patients and 53 healthy controls: 165 who had received seasonal influenza vaccine and 101 who had not. IBD medications included infliximab, thiopurines, infliximab and thiopurine combination therapy, ustekinumab, vedolizumab, or tofacitinib. The primary outcome was antibody responses against influenza/A H3N2 and A/H1N1, compared to controls, adjusting for age, prior vaccination, and interval between vaccination and sampling.
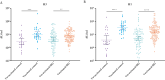
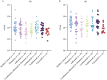
Results: Lower antibody responses against influenza A/H3N2 were observed in patients on infliximab (geometric mean ratio 0.35 [95% confidence interval 0.20-0.60], p = 0.0002), combination of infliximab and thiopurine therapy (0.46 [0.27-0.79], p = 0.0050), and tofacitinib (0.28 [0.14-0.57], p = 0.0005) compared to controls. Lower antibody responses against A/H1N1 were observed in patients on infliximab (0.29 [0.15-0.56], p = 0.0003), combination of infliximab and thiopurine therapy (0.34 [0.17-0.66], p = 0.0016), thiopurine monotherapy (0.46 [0.24-0.87], p = 0.017), and tofacitinib (0.23 [0.10-0.56], p = 0.0013). Ustekinumab and vedolizumab were not associated with reduced antibody responses against A/H3N2 or A/H1N1. Vaccination in the previous year was associated with higher antibody responses to A/H3N2. Vaccine-induced anti-SARS-CoV-2 antibody concentration weakly correlated with antibodies against H3N2 [r = 0.27; p = 0.0004] and H1N1 [r = 0.33; p < 0.0001].
Conclusions: Vaccination in both the 2020-2021 and 2021-2022 seasons was associated with significantly higher antibody responses to influenza/A than no vaccination or vaccination in 2021-2022 alone. Infliximab and tofacitinib are associated with lower binding antibody responses to influenza/A, similar to COVID-19 vaccine-induced antibody responses.
Keywords: JAK-inhibitor; anti-TNF; humoral immunity; immunisation.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
JLA reports he has received travel expense support from Takeda outside the submitted work. NAK reports grants from AbbVie, Biogen, Celgene, Celltrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; personal fees from Allergan, Celltrion, Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tilllotts, and Galapagos; and support for attending meetings from AbbVie, Falk, and Janssen, outside the submitted work. LH has received travel expense support from Abbvie. AS has received travel expense support from Janssen. SS reports grants from Takeda, AbbVie, Tillots Pharma, Janssen, Pfizer, and Biogen, and personal fees from Takeda, AbbVie, Janssen, Pharmacocosmos, Biogen, Pfizer, Tillots Pharma, and Falk Pharma, outside the submitted work. ALH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, AZ, Atlantic, Bristol Myers Squibb, Celltrion, Falk, Galapagos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda; participation on the Global Steering Committee for Genentech; support for attending meetings from AbbVie, Takeda, and Janssen; and participation on a data safety monitoring board or advisory board for AbbVie, AZ, Atlantic, Bristol Myers Squibb, Galapogos, Janssen, Pfizer, and Takeda. PMI reports grants from Celltrion, Takeda, MSD, Pfizer, and Galapagos, and personal fees from Celltrion, Takeda, Pfizer, Galapagos, Gilead, AbbVie, Janssen, Bristol Myers Squibb, Lilly, and Arena, outside the submitted work. MP receives unrestricted educational grants from Pfizer for genetic analyses to support the IBD BioResource and speaker fees from Janssen. GRJ has received grants from the Wellcome Trust and ECCO; speaker fees from Takeda, Ferring, and Janssen; and support for attending meetings or travel from Ferring. KK reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Ferring; support for attending meetings or travel from Janssen and Takeda; and participation on a data safety monitoring board or advisory board for Janssen and PredictImmune. KVP reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, DrFalk, Janssen, PreddictImmune, and Takeda; support for attending meetings or travel from AbbVie, Ferring, Janssen, and Tillotts; and participation on a data safety monitoring board or advisory board for AbbVie, Galapagos, and Janssen. AJK reports consulting fees from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer and Takeda; support for attending meetings or travel from Janssen, Tillotts, and Norgine; and participation in a data safety monitoring board or advisory board for AbbVie. LCH reports support for attending meetings or travel from AbbVie. CWL reports a Future Leaders Fellow award from UK Research and Innovation; personal consulting fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, and Iterative Scopes; institutional consulting fees from Trellus Health; personal fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk; and support for attending meetings from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk. RJB and DMA are members of the Global T cell Expert Consortium and have consulted for Oxford Immunotec outside the submitted work. JRG reports grants from F Hoffmann-La Roche, Biogen, Celltrion Healthcare, and Galapagos, and non-financial support from Immundiagnostik during the study. TA reports grant funding from Pfizer to his institution to deliver this study; grants from Celltrion, Roche, Takeda, Biogen, and Galapagos; and honoraria for lectures from Takeda and Roche, outside the submitted work. KMP is the chief, principal, or co-investigator for vaccine clinical trials and experimental medicine studies [NCT05007275, NCT04753892, EudraCT 2020-001646-20, NCT04400838, NCT04324606, EudraCT 2017-004610-26, NCT03970993, NCT03816137], is a member of the data safety monitoring board for NCT05249829, has received a fee for speaking from Seqirus and Sanofi Pasteur, and has research funding from the Chan Zuckerberg Initiative, the MRC/UKRI, the Vaccine Task Force, and NIHR Imperial BRC outside the submitted work. NP is the principal investigator on the research grant from Pfizer that funded the VIP study; has received research grants from Bristol Myers Squibb outside the submitted work; reports personal fees from Takeda, Janssen, Pfizer, Galapagos, Bristol Myers Squibb, AbbVie, Roche, Lilly, Allergan, and Celgene, Astra Zeneca outside the submitted work; and has served as a speaker or advisory board member for AbbVie, Allergan, Bristol Myers Squibb, Celgene, Falk, Ferring, Janssen, Pfizer, Tillotts, Takeda, and Vifor Pharma. All other authors declare no competing interests.
Figures


References
-
- Alexander JL, Liu Z, Muñoz Sandoval D, et al.; VIP study investigators. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol 2022;7:1005–15. - PMC - PubMed
-
- Liu Z, Alexander JL, Lin KW, Ahmad T, Pollock KM, Powell N; VIP Study Investigators. Infliximab and tofacitinib attenuate neutralizing antibody responses against SARS-CoV-2 ancestral and omicron variants in inflammatory bowel disease patients after 3 doses of COVID-19 vaccine. Gastroenterology 2023;164:300–303.e3. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

