Lithiation Gradients and Tortuosity Factors in Thick NMC111-Argyrodite Solid-State Cathodes
- PMID: 37941794
- PMCID: PMC10629242
- DOI: 10.1021/acsenergylett.2c02699
Lithiation Gradients and Tortuosity Factors in Thick NMC111-Argyrodite Solid-State Cathodes
Abstract
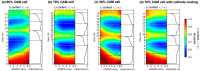
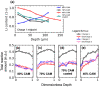
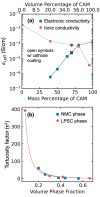
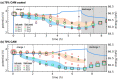
Achieving high energy density in all-solid-state lithium batteries will require the design of thick cathodes, and these will need to operate reversibly under normal use conditions. We use high-energy depth-profiling X-ray diffraction to measure the localized lithium content of Li1-xNi1/3Mn1/3Co1/3O2 (NMC111) through the thickness of 110 μm thick composite cathodes. The composite cathodes consisted of NMC111 of varying mass loadings mixed with argyrodite solid electrolyte Li6PS5Cl (LPSC). During cycling at C/10, substantial lithiation gradients developed, and varying the NMC111 loading altered the nature of these gradients. Microstructural analysis and cathode modeling showed this was due to high tortuosities in the cathodes. This was particularly true in the solid electrolyte phase, which experienced a marked increase in tortuosity factor during the initial charge. Our results demonstrate that current distributions are observed in sulfide-based composites and that these will be an important consideration for practical design of all-solid-state batteries.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Janek J.; Zeier W. G. "A solid future for battery development.". Nature Energy 2016, 1 (9), 16141.10.1038/nenergy.2016.141. - DOI
-
- Parejiya A.; Amin R.; Dixit M. B.; Essehli R.; Jafta C. J.; Wood D. L. III; Belharouak I. ″Improving Contact Impedance via Electrochemical Pulses Applied to Lithium–Solid Electrolyte Interface in Solid-State Batteries.″. ACS Energy Letters 2021, 6 (10), 3669–3675. 10.1021/acsenergylett.1c01573. - DOI
-
- Kim J. Y.; Park J.; Lee M. J.; Kang S. H.; Shin D. O.; Oh J.; Kim J.; Kim K. M.; Lee Y.-G.; Lee Y. M. ″Diffusion-dependent graphite electrode for all-solid-state batteries with extremely high energy density.″. Acs Energy Letters 2020, 5 (9), 2995–3004. 10.1021/acsenergylett.0c01628. - DOI
-
- Bachman J. C.; Muy S.; Grimaud A.; Chang H.-H.; Pour N.; Lux S. F.; Paschos O.; Maglia F.; Lupart S.; Lamp P.; et al. ″Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction.″. Chem. Rev. 2016, 116 (1), 140–162. 10.1021/acs.chemrev.5b00563. - DOI - PubMed
LinkOut - more resources
Full Text Sources
