Development of an acute ovine model of polycystic ovaries to assess the effect of ovarian denervation
- PMID: 37941906
- PMCID: PMC10628477
- DOI: 10.3389/fendo.2023.1285269
Development of an acute ovine model of polycystic ovaries to assess the effect of ovarian denervation
Abstract
Introduction: Polycystic ovary syndrome (PCOS) seems to be associated with increased ovarian sympathetic nerve activity and in rodent models of PCOS reducing the sympathetic drive to the ovary, through denervation or neuromodulation, improves ovulation rate. We hypothesised that sympathetic nerves work with gonadotropins to promote development and survival of small antral follicles to develop a polycystic ovary phenotype.
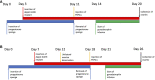
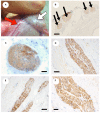
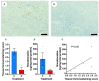
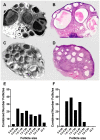
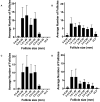
Methods: Using a clinically realistic ovine model we showed a rich sympathetic innervation to the normal ovary and reinnervation after ovarian transplantation. Using needlepoint diathermy to the nerve plexus in the ovarian vascular pedicle we were able to denervate the ovary resulting in reduced intraovarian noradrenaline and tyrosine hydroxylase immunostained sympathetic nerves. We developed an acute polycystic ovary (PCO) model using gonadotrophin releasing hormone (GnRH) agonist followed infusion of follicle stimulating hormone (FSH) with increased pulsatile luteinising hormone (LH). This resulted in increased numbers of smaller antral follicles in the ovary when compared to FSH infusion suggesting a polycystic ovary.
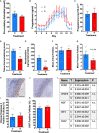
Results: Denervation had no effect of the survival or numbers of follicles in the acute PCO model and did not impact on ovulation, follicular and luteal hormone profiles in a normal cycle.
Discussion: Although the ovary is richly inervated we did not find evidence for a role of sympathetic nerves in ovarian function or small follicle growth and survival.
Keywords: follicle; gonadotrophin; polycystic ovary syndrome; sympathetic nerve; tyrosine hydroxylase.
Copyright © 2023 Duncan, Nicol, O’Hare, Witherington, Miranda, Campbell, Thomas and Rae.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

