Multifaceted personality and roles of heme enzymes in industrial biotechnology
- PMID: 37942054
- PMCID: PMC10630290
- DOI: 10.1007/s13205-023-03804-8
Multifaceted personality and roles of heme enzymes in industrial biotechnology
Abstract
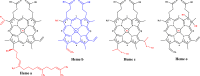
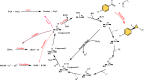
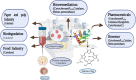
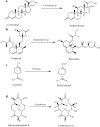
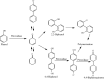
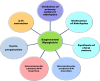
Heme enzymes are the most prominent category of iron-containing metalloenzymes with the capability of catalyzing an astonishingly wide range of reactions like epoxidation, hydroxylation, demethylation, desaturation, reduction, sulfoxidation, and decarboxylation. Various enzymes in this category are P450s, heme peroxidases, catalases, myoglobin, cytochrome C, and others. Besides this, the natural promiscuity and amenability of these enzymes to protein engineering and evolution have also added several non-native reactions such as C-H, N-H, S-H insertions, cyclopropanation, and other industrially important reactions to their capabilities. Surprisingly, all of these reactions and their wide substrate scopes are attributed to changes in the active site scaffold of different heme enzymes as the center of all enzymes is constituted by a porphyrin ring containing iron. Multiple prominent research groups across the world, including 2018, Nobel Laureate Frances Arnold's group, have shown keen interest in engineering and evolving these enzymes for utilizing their industrial potential. Besides engineering the active site, researchers have also explored the possibility of these enzymes catalyzing non-native reactions by replacing the center porphyrin ring with other cofactors or by changing the iron in the porphyrin ring with other metal ions along with engineering the active site and thereby creating novel artificial metalloenzymes. Thus, in this mini-review from our group, for the first time, we are trying to catalog various activities catalyzed by heme enzymes and their engineered variants and their active usage in various industries along with shedding light on their potential for use in various applications in the future.
Keywords: Artificial metalloenzymes; Biocatalyst; Catalase; Green chemistry; Heme; Industrial biotechnology; Myoglobin; P450; Peroxidase.
© King Abdulaziz City for Science and Technology 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures
References
-
- Akyilmaz E, Kozgus O. Determination of calcium in milk and water samples by using catalase enzyme electrode. Food Chemistry. 2009;115:347–351. doi: 10.1016/j.foodchem.2008.11.075. - DOI
Publication types
LinkOut - more resources
Full Text Sources

