Vaccine efficacy against SARS-CoV-2 for Pfizer BioNTech, Moderna, and AstraZeneca vaccines: a systematic review
- PMID: 37942238
- PMCID: PMC10628441
- DOI: 10.3389/fpubh.2023.1229716
Vaccine efficacy against SARS-CoV-2 for Pfizer BioNTech, Moderna, and AstraZeneca vaccines: a systematic review
Abstract
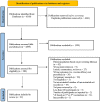
The purpose of this systematic review was to report on the vaccine efficacy (VE) of three SARS-CoV-2 vaccines approved by Health Canada: Pfizer BioNTech, Moderna, and AstraZeneca. Four databases were searched for primary publications on population-level VE. Ninety-two publications matched the inclusion criteria, and the extracted data were separated by vaccine type: mRNA vaccines (Pfizer and Moderna) and the AstraZeneca vaccine. The median VE for PCR-positive patients and various levels of clinical disease was determined for the first and second doses of both vaccine types against multiple SARS-CoV-2 variants. The median VE for PCR-positive infections against unidentified variants from an mRNA vaccine was 64.5 and 89%, respectively, after one or two doses. The median VE for PCR-positive infections against unidentified variants from the AstraZeneca vaccine was 53.4 and 69.6%, respectively, after one or two doses. The median VE for two doses of mRNA for asymptomatic, symptomatic, and severe infection against unidentified variants was 85.5, 93.2, and 92.2%, respectively. The median VE for two doses of AstraZeneca for asymptomatic, symptomatic, and severe infection against unidentified variants was 69.7, 71, and 90.2%, respectively. Vaccine efficacy numerically increased from the first to the second dose, increased from the first 2 weeks to the second 2 weeks post-vaccination for both doses, but decreased after 4 months from the second dose. Vaccine efficacy did not differ by person's age.
Keywords: AstraZeneca vaccine; COVID-19; SARS-CoV-2; mRNA vaccines; systematic review; vaccine efficacy.
Copyright © 2023 Reynolds, Dewey, Asfour and Little.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization (WHO) . Coronavirus (COVID-19) Dashboard. (2022). Available online at: https://covid19.who.int/ (accessed January 28, 2023).
-
- U.S. Food and Drug Administration (FDA) . FDA Takes Key Action in Fight Against COVID-19 by Issuing Emergency use Authorization for First COVID-19 Vaccine. (2020). Available online at: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action... (accessed July 13, 2022).
-
- Government of Canada . COVID-19 Vaccines: Authorized Vaccines. (2022). Available online at: https://www.canada.ca/en/health-canada/services/drugs-health-products/co... (accessed July 13, 2022).
-
- Pfizer . Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. (2020). Available online at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-an... (accessed July 13, 2022).
-
- Moderna . Moderna Announces Publication of Results from the Pivotal Phase 3 Trial of the Moderna COVID-19 Vaccine in The New England Journal of Medicine. (2020). Available online at: https://investors.modernatx.com/news/news-details/2020/Moderna-Announces... (accessed July 13, 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous