Fibroblasts alter the physical properties of dermal ECM-derived hydrogels to create a pro-angiogenic microenvironment
- PMID: 37942422
- PMCID: PMC10628774
- DOI: 10.1016/j.mtbio.2023.100842
Fibroblasts alter the physical properties of dermal ECM-derived hydrogels to create a pro-angiogenic microenvironment
Abstract
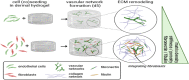
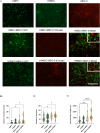
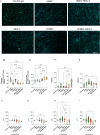
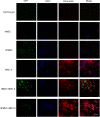
This study aimed to investigate the impact of fibroblasts (MRC-5) on the extracellular matrix (ECM) microenvironment of endothelial cells (ECs) during the vascularization of skin-derived ECM hydrogel in vitro. Two types of ECs were studied: human dermal microvascular endothelial cells (HMEC) and human pulmonary microvascular endothelial cells (HPMEC). Results showed that the presence of MRC-5 fibroblasts increased the stiffness of the hydrogel and led to larger fiber diameters and increased porosity. Extensive collagen fiber remodeling occurred in the ECM hydrogel with MRC-5 fibroblasts. Additionally, higher levels of fibulin-1 and fibronectin were deposited in the hydrogel when co-cultured with MRC-5 fibroblasts. These findings suggest that MRC-5 fibroblasts play a role in modifying the ECM microenvironment, promoting vascularization through dynamic ECM remodeling.
Keywords: Biomechanics; Collagen; ECM hydrogel; Endothelial cells; Extracellular matrix; Fibronectin; Vascularization.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources

