Recent synthetic strategies of spiro-azetidin-2-one, -pyrrolidine, -indol(one) and -pyran derivatives-a review
- PMID: 37942448
- PMCID: PMC10628897
- DOI: 10.1039/d3ra06054c
Recent synthetic strategies of spiro-azetidin-2-one, -pyrrolidine, -indol(one) and -pyran derivatives-a review
Abstract
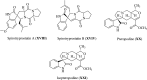
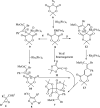
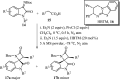
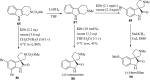
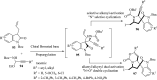
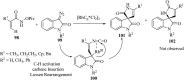
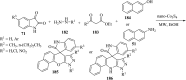
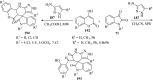
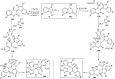
Spiro-heterocycles have received special attention in medicinal chemistry because of their promising biological activity. Over the years, many synthetic methodologies have been established for the construction of spirocyclic compounds. Spiro heterocycles such as spiro-azetidin-2-one, -pyrrolidine, -indol(one) and -pyran derivatives have been found to exhibit diversified biological and pharmacological activity in addition to their therapeutic properties. In view of these facts, we decided in this review to present representative synthetic approaches of the aforementioned spiro heterocycles, especially in the past 20 years.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
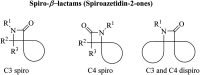

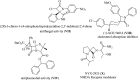
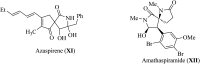
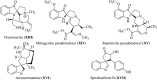


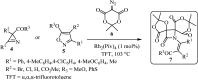
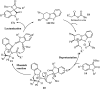

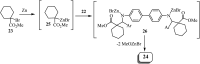
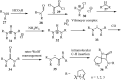
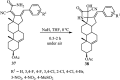
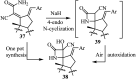

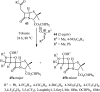
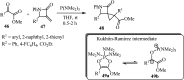
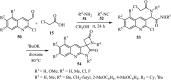
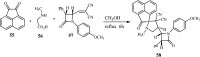

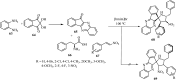

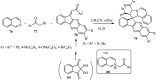
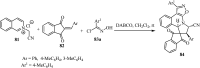

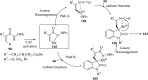
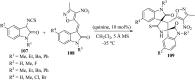

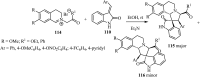
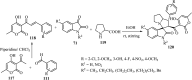
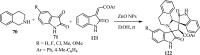
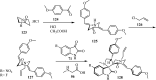
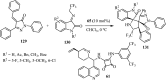
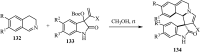
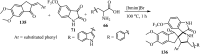
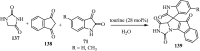
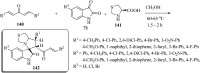
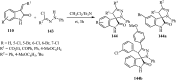
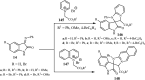
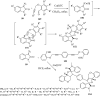
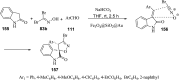
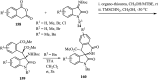




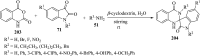
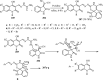
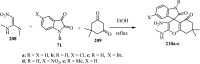
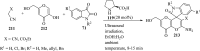




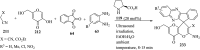
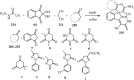
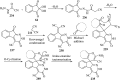


References
-
- Zheng Y. Tice C. M. Singh S. B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014;24:3673–3682. - PubMed
-
- Zheng Y.-J. Tice C. M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discovery. 2016;11:831–834. - PubMed
-
- Molvi K. I. Haque N. Awen B. Z. S. Zameeruddin M. Synthesis of spiro compounds as medicinal agents; New opportunities for drug design and discovery. Part I: A Review. World J. Pharm. Pharm. Sci. 2014;3:536–563.
-
- Zhou L.-M. Qu R.-Y. Yang G.-F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discovery. 2020;15:603–625. - PubMed
-
- Alves A. J. S. Alves N. G. Soares M. I. L. Pinho e Melo T. M. V. D. Strategies and methodologies for the construction of spiro-γ-lactams: An update. Org. Chem. Front. 2021;8:3543–3593.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

