Recent achievements in the synthesis of benzimidazole derivatives
- PMID: 37942457
- PMCID: PMC10628531
- DOI: 10.1039/d3ra05960j
Recent achievements in the synthesis of benzimidazole derivatives
Abstract
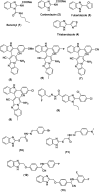
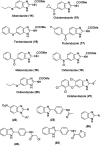
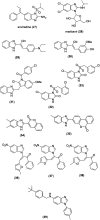
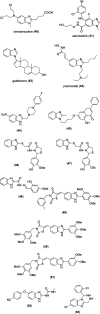
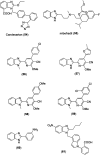
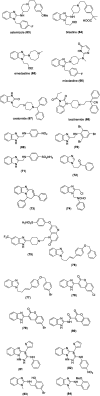
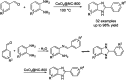
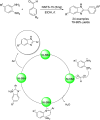
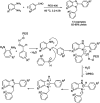
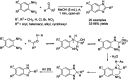
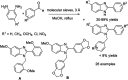
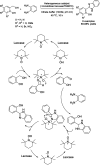
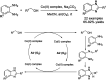
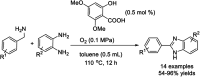
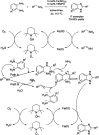
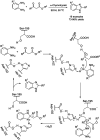
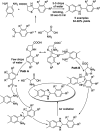
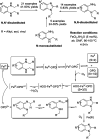
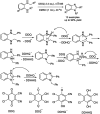
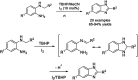
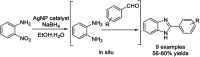
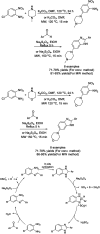
Benzimidazoles are a class of heterocyclic compounds in which a benzene ring is fused to the 4 and 5 positions of an imidazole ring. Benzimidazole refers to the parent compound, while benzimidazoles are a class of heterocyclic compounds having similar ring structures, but different substituents. Benzimidazole derivatives possess a wide range of bioactivities including antimicrobial, anthelmintic, antiviral, anticancer, and antihypertensive activities. Many compounds possessing a benzimidazole skeleton have been employed as drugs in the market. The application of benzimidazoles in other fields has also been documented. The synthesis of benzimidazole derivatives has attracted much attention from chemists and numerous articles on the synthesis of this class of heterocyclic compound have been reported over the years. The condensation between 1,2-benzenediamine and aldehydes has received intensive interest, while many novel methods have been developed. In this article, we will give a comprehensive review of studies on the synthesis of benzimidazole, which date back to 2013. We have also tried to describe reaction mechanisms as much as we can. The work might be useful for chemists who work in the synthesis of heterocycles or drug chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

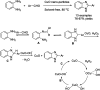

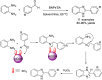
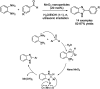
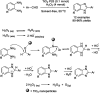
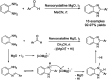

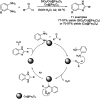
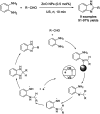
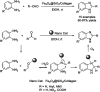
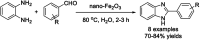
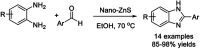
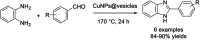
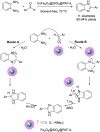


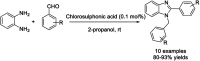

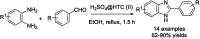
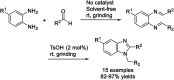
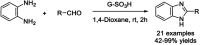

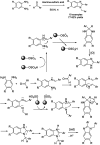
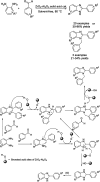
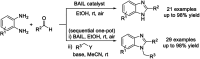
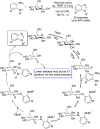

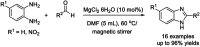
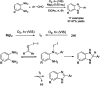


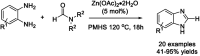
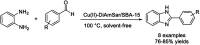
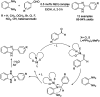
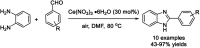

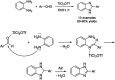
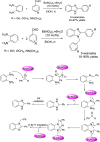





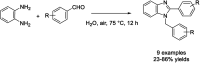
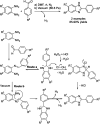

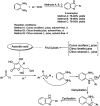



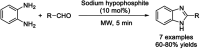
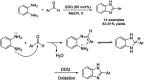

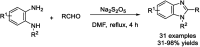
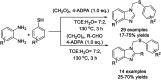
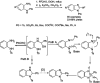
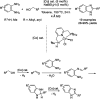



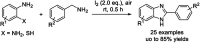
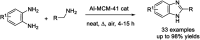
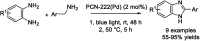
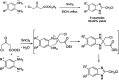
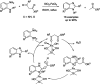
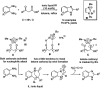
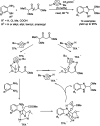









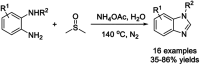
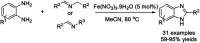

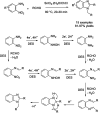
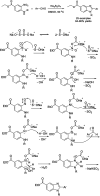
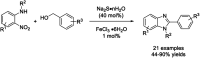
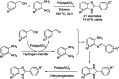
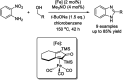
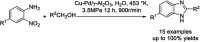
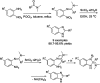
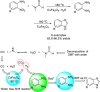
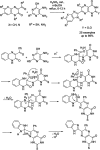
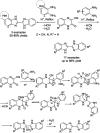
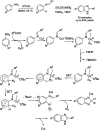
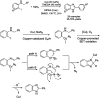

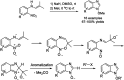
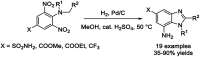
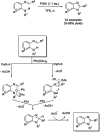

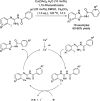
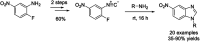
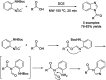
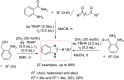

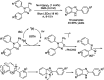

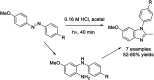




References
-
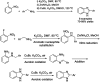
- Kathrotiya H. G. Patel M. P. J. Chem. Sci. 2013;125:993–1001.
-
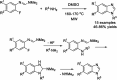
- Vasantha K. Basavarajaswamy G. Vaishali Rai M. Boja P. Pai V. R. Shruthi N. Bhat M. Bioorg. Med. Chem. Lett. 2015;25:1420–1426. - PubMed
-
- Yadav S. Narasimhan B. Lim S. M. Ramasamy K. Vasudevan M. Shah S. A. A. Mathur A. J. Basic Appl. Sci. 2018;5:100–109.
-
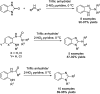
- Shaikh I. N. Hosamani K. M. Kurjogi M. M. Arch. Pharm. 2018;351:1700205. - PubMed
-
- Faruk A. Biplab K. D. Kamal S. Arpita C. Pallab K. Int. J. Drug Res. Technol. 2014;4:31–38.
Publication types
LinkOut - more resources
Full Text Sources

