Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling
- PMID: 37942583
- PMCID: PMC10730136
- DOI: 10.1080/19490976.2023.2274124
Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling
Abstract
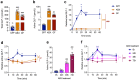
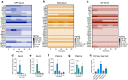
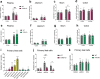
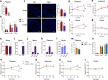
The gut microbiota interacts with intestinal epithelial cells through microbial metabolites to regulate the release of gut hormones. We investigated whether the gut microbiota affects the postprandial glucagon-like peptide-1 (GLP-1) response using antibiotic-treated mice and germ-free mice. Gut microbiome depletion completely abolished postprandial GLP-1 response in the circulation and ileum in a lipid tolerance test. Microbiome depletion did not influence the GLP-1 secretory function of primary ileal cells in response to stimulators in vitro, but dramatically changed the postprandial dynamics of endogenous bile acids, particularly ω-muricholic acid (ωMCA) and hyocholic acid (HCA). The bile acid receptor Takeda G protein-coupled receptor 5 (TGR5) but not farnesoid X receptor (FXR), participated in the regulation of postprandial GLP-1 response in the circulation and ileum, and ωMCA or HCA stimulated GLP-1 secretion via TGR5. Finally, fecal microbiota transplantation or ωMCA and HCA supplementation restored postprandial GLP-1 response. In conclusion, gut microbiota is indispensable for maintaining the postprandial GLP-1 response specifically in the ileum, and bile acid (ωMCA and HCA)-TGR5 signaling is involved in this process. This study helps to understand the essential interplay between the gut microbiota and host in regulating postprandial GLP-1 response and opens the foundation for new therapeutic targets.
Keywords: GLP-1; Microbiome; TGR5; bile acid; diabetes; ileum.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
