Ascorbate protects human kidney organoids from damage induced by cell-free hemoglobin
- PMID: 37942584
- PMCID: PMC10695115
- DOI: 10.1242/dmm.050342
Ascorbate protects human kidney organoids from damage induced by cell-free hemoglobin
Abstract
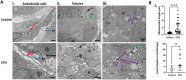
Sepsis-associated acute kidney injury is associated with high morbidity and mortality in critically ill patients. Cell-free hemoglobin (CFH) is released into the circulation of patients with severe sepsis and the levels of CFH are independently associated with mortality. CFH treatment increased cytotoxicity in the human tubular epithelial cell line HK-2. To better model the intact kidney, we cultured human kidney organoids derived from induced pluripotent stem cells. We treated human kidney organoids grown using both three-dimensional and transwell protocols with CFH for 48 h. We found evidence for increased tubular toxicity, oxidative stress, mitochondrial fragmentation, endothelial cell injury and injury-associated transcripts compared to those of the untreated control group. To evaluate the protective effect of clinically available small molecules, we co-treated CFH-injured organoids with ascorbate (vitamin C) or acetaminophen for 48 h. We found significantly decreased toxicity, preservation of endothelial cells and reduced mitochondrial fragmentation in the group receiving ascorbate following CFH treatment. This study provides direct evidence that ascorbate or ascorbic acid protects human kidney cells from CFH-induced damage such as that in sepsis-associated acute kidney injury.
Keywords: Acute kidney injury; Cell-free hemoglobin; Induced pluripotent stem cells; Kidney; Organoids; Sepsis.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures







References
-
- Agyemang, A. A., Kvist, S. V., Brinkman, N., Gentinetta, T., Illa, M., Ortenlöf, N., Holmqvist, B., Ley, D. and Gram, M. (2021). Cell-free oxidized hemoglobin drives reactive oxygen species production and pro-inflammation in an immature primary rat mixed glial cell culture. J. Neuroinflammation 18, 42. 10.1186/s12974-020-02052-4 - DOI - PMC - PubMed
-
- Barber, B. E., Grigg, M. J., Piera, K. A., William, T., Cooper, D. J., Plewes, K., Dondorp, A. M., Yeo, T. W. and Anstey, N. M. (2018). Intravascular haemolysis in severe Plasmodium knowlesi malaria: association with endothelial activation, microvascular dysfunction, and acute kidney injury. Emerg. Microbes Infect. 7, 1-10. 10.1038/s41426-018-0105-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R25 GM134979/GM/NIGMS NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- T32 ES007028/ES/NIEHS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- 5T32ES007028/NH/NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 HL158906/HL/NHLBI NIH HHS/United States
- CA68485/NH/NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- I01 BX004845/BX/BLRD VA/United States
- R25GM134979/NH/NIH HHS/United States
- R01 HL164937/HL/NHLBI NIH HHS/United States
- EY08126/NH/NIH HHS/United States
- DK59637/NH/NIH HHS/United States
- DK584047/NH/NIH HHS/United States
- DK20593/NH/NIH HHS/United States
- P30 EY08126/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

