Nebuliser systems for drug delivery in cystic fibrosis
- PMID: 37942828
- PMCID: PMC10633867
- DOI: 10.1002/14651858.CD007639.pub3
Nebuliser systems for drug delivery in cystic fibrosis
Abstract
Background: Nebuliser systems are used to deliver medications to the lungs, to control the symptoms and the progression of lung disease in people with cystic fibrosis (CF). There are many different nebulised-medications prescribed for people with CF and there are many different types of nebuliser systems. Some of these nebulised medications are licenced for, and can be taken via only one type of nebuliser system; some are licensed for, and can be taken via more than one type of nebuliser system. This is an update to a previous systematic review.
Objectives: To assess the time efficiency, effectiveness, safety, cost and impact of use (e.g. burden of care, adherence, quality of life (QoL)) of different nebuliser systems, when used with different inhaled medications for people with CF.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, handsearching of relevant journals and abstract books containing conference proceedings. We searched the reference lists of each study for additional publications and approached the manufacturers of both nebuliser systems and nebulised medications for published and unpublished data. We also searched online trial registries. Date of the most recent search: 9 August 2023.
Selection criteria: Randomised controlled trials (RCTs) or quasi-RCTs comparing nebuliser systems, including conventional nebulisers, vibrating mesh technology (VMT) systems, adaptive aerosol delivery (AAD) systems and ultrasonic nebuliser systems.
Data collection and analysis: Two review authors independently assessed studies for inclusion. They also independently extracted data and assessed the risk of bias. A third review author assessed studies where agreement could not be reached. They assessed the certainty of the evidence using GRADE.
Main results: The search identified 216 studies with 33 of these (2270 participants) included in the review. These studies compared the delivery of tobramycin, colistin, dornase alfa, hypertonic saline and other solutions through the different nebuliser systems in children and adults with CF. This review demonstrates variability in the delivery of medication depending on the nebuliser system used. The certainty of the evidence ranged from low to very low. Some conventional nebuliser systems providing higher flows, higher respirable fractions, and smaller particles decrease treatment time, increase deposition (the amount of drug reaching the lung), and may be preferred by people with CF, as compared to other conventional nebuliser systems providing lower flows, lower respirable fractions and larger particles. Newer nebuliser systems using AAD, or VMT (or both) reduce treatment time compared to conventional systems. Deposition (as a percentage of priming dose) with AAD is greater than with conventional systems. VMT systems may give greater deposition than conventional systems when measuring sputum levels. The available data indicate that these newer systems are safe when used with an appropriate priming dose, which may be different to the priming dose used for conventional systems. There is an indication that adherence is maintained or improved and that individuals prefer AAD or VMT systems, but also that some nebuliser systems using VMT may be subject to increased system failures. There is limited, unclear evidence on the impact of different nebuliser systems on lung function and a lack of data on the impact of different nebuliser systems on our outcomes of quality of life (QoL), adverse effects, respiratory exacerbations and related implications, adherence, satisfaction, cost and device reliability.
Authors' conclusions: Newer technologies e.g. AAD and VMT have advantages over conventional systems in terms of treatment time, deposition as a percentage of priming dose, preference and adherence. Data are lacking for all varieties of medications which are used in CF care, including different inhaled antibiotics or hypertonic saline, with all delivery (nebuliser system) possibilities. Long-term RCTs are needed to evaluate different nebuliser systems to determine patient-focused outcomes (such as QoL and burden of care), safe and effective dosing levels of a wide variety of medications, clinical outcomes (such as hospitalisations and need for antibiotics), and an economic evaluation of their use. There are insufficient data to establish whether one nebuliser system is better than another overall. Clinicians should be aware of the variability in the performance of different nebuliser systems, compatibility with specific nebulised medication, and they must work with their patients to choose the best nebuliser system for each individual. This is likely to be an ongoing process as the needs and circumstances of each individual change over time.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
GS declares she was a recipient of a Health Education England/National Institute of Health Research Clinical Doctoral Fellowship from January 2016 to April 2022; however, this does not represent a potential conflict of interest in this review.
LM declares no potential conflicts of interest.
CB declares she has received consultancy, speaker honoraria and educational grants from Chiesi Farmaceutici, speaker honoraria from Gilead Sciences, consultancy and speaker honoraria from Insmed, Inc., educational grants from PARI Medical, an educational grant from Teva Pharmaceutical industries, and an educational grant from Zambon.
All authors have worked on developing national guidelines ‐
Figures
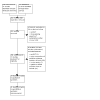









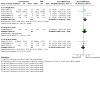










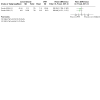








































Update of
-
Nebuliser systems for drug delivery in cystic fibrosis.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD007639. doi: 10.1002/14651858.CD007639.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2023 Nov 9;11:CD007639. doi: 10.1002/14651858.CD007639.pub3. PMID: 23633344 Updated.
References
References to studies included in this review
Brand 2006 {published data only}
-
- Brand P, Häussermann S, Mullinger B, Fischer A, Wachall B, Stegemann J. Reduction of drug-dose and therapy-costs in the inhalation therapy with high dose tobramycin. Journal of Cystic Fibrosis 2006;5(Suppl 1):S40. [CFGD REGISTER: DT35]
Byrne 2003 {published data only}
-
- Spencer DA, Byrne N, Keavey PM, Snowdon V, Hawkins T. Radionuclide assessment of lung deposition of nebulised antibiotics in patients with cystic fibrosis. In: 24th European Cystic Fibrosis Conference; 2001 June 6-9; Vienna, Austria. 2001:P75. [CFGD REGISTER: DT22a]
-
- Spencer DA, Byrne N, Keavey PM, Snowdon V, Hawkins T. Radionuclide assessment of lung deposition of nebulised antibiotics in patients with cystic fibrosis. Pediatric Pulmonology 2001;32 (Suppl 22):314-5. [CFGD REGISTER: DT22c]
-
- Spencer DA, Byrne N, Perry J, Gould K. Microbiological assessment of antibiotic delivery using two nebuliser systems in patients with cystic fibrosis. In: 24th European Cystic Fibrosis Conference; 2001 June 6-9; Vienna, Austria. 2001:P219. [CFGD REGISTER: DT22b]
Clavel 2007 {published data only}
-
- Clavel A, Boulamery A, Bosdure E, Luc C, Lanteaume A, Gorincour G, et al. Nebulisers comparison with inhaled tobramycin in young children with cystic fibrosis. Journal of Cystic Fibrosis 2007;6(2):137-43. [CFGD REGISTER: DT36] - PubMed
Coates 2011 {published data only}
-
- Coates AL, Denk O, Leung K, Ribeiro N, Chan J, Green M, et al. Higher tobramycin concentration and vibrating mesh technology can shorten antibiotic treatment time in cystic fibrosis. Pediatric Pulmonology 2011;46(4):401-8. [CFGD REGISTER: PI241b] - PubMed
-
- Denk O, Caotes AL, Keller M, Leung K, Green M, Chan J, Ribeiro N, Charron M. Lung delivery of a new tobramycin nebuliser solution (150mg/1.5ml) by an investigational eFlow® nebuliser is equivalent to TOBI® but in a fraction of time. Journal of Cystic Fibrosis 2009;8 (Suppl 2):S66, Abstract no: 264. [CFGD REGISTER: PI241c]
-
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In-vivo data support equivalent therapeutic efficacay of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS®. Journal of Cystic Fibrosis 2010;9 (Suppl 1):S22, Abstract no: 84. [CFGD REGISTER: PI241a]
Devadason 1997 {published data only}
-
- Devadason SG, Everard ML, Linto JM, Le Souef PN. Comparison of drug delivery from conventional versus "venturi" nebulisers. European Respiratory Journal 1997;10(11):2479-83. [CFGD REGISTER: DT15] - PubMed
Devadason 2001 {published data only}
-
- Devadason SG, Huang T, Walker SL, Troedson R, Le Souef PN. Lung deposition of rhDNase in children with cystic fibrosis using the Halolite adaptive aerosol delivery (AAD) systems and Pari LC+ nebulizer. In: 24th European Cystic Fibrosis Conference; 2001 June 6-9; Vienna, Austria. 2001:P84. [CFGD REGISTER: DT23]
Dodd 2002 {published data only}
-
- Conway SP, Dodd ME, Marsden RJ, Paul EA, Weller PH. Comparison of compliance in Cystic Fibrosis patients using either a Halolite adaptive aerosol delivery (AAD) system of a conventional high output nebuliser system. Journal of Cystic Fibrosis 2002;1 (Suppl 1):S67:P161. [CFGD REGISTER: DT28g]
-
- Dodd M, Conway S, Marsden R, Paul E, Weller P. Interaction between bronchodilators and nebuliser device in Cystic Fibrosis patients taking Colistin using a halolite adaptive aerosol delivery (AAD) system compared to a high output conventional nebuliser system. Journal of Cystic Fibrosis 2002;1 (Suppl 1):S106. [CFGD REGISTER: DT28b]
-
- Dodd ME, Conway S, Marsden R, Weller P. Interaction between aerosol delivery system and bronchodilators in CF patients taking colistin. Pediatric Pulmonology 2002;34 (Suppl 24):321. [CFGD REGISTER: DT28d]
-
- Dodd ME, Conway S, Marsden RJ, Weller PH. Interaction between aerosol delivery system and bronchodilators in CF patients taking colistin. Thorax 2002;57 (Suppl 3):iii39. [CFGD REGISTER: DT28e]
-
- Marsden R, Conway S, Dodd M, Edenborough F, Paul E, Rigby A, et al. A multi-centre, randomised study comparing the halolite adaptive aerosol delivery system with a high output nebuliser system in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1 (Suppl 1):S105-6: P120. [CFGD REGISTER: DT28a]
Dopfer 2007 {published data only}
-
- Dopfer R, Brand P, Muellinger B, Hunger T, Haeussermann S, Meyer T, et al. Inhalation of tobramycin in patients with cystic fibrosis: comparison of two methods. Journal of Physiology and Pharmacology 2007;58(Suppl 5):141-54. [CFGD REGISTER: DT32b] - PubMed
-
- Haeussermann S, Dopfer R, Brand P, Muellinger B, Meyer TH, Scheuch G. Tobramycin serum concentrations after conventional and controlled inhalations in patients with cystic fibrosis. In: American Thoracic Society International Conference; 2006 May 19-24; California, USA. 2006:A407. [CFGD REGISTER: DT32a]
Eisenberg 1997 {published data only}
-
- Eisenberg J, Pepe M, Williams-Warren J, Vasiliev M, Montgomery AB, Smith AL, et al. A comparison of peak sputum concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Chest 1997;111(4):955-62. [CFGD REGISTER: PI117] - PubMed
Elkins 2006 {published data only}
-
- Elkins MR, Bye PT. Comparison of Pari LC-Star and -Plus nebulisers delivering 2.5mg recombinant human deoxyribonuclease (rhDNase). Journal of Cystic Fibrosis 2006;5 (Suppl 1):S42. [CFGD REGISTER: DT34]
Fiel 1995 {published data only}
-
- Fiel SB, Fuchs HJ, Johnson C, Gonda I, Clark AR. Comparison of three jet nebulizer aerosol delivery systems used to administer recombinant human DNase I to patients with cystic fibrosis. Chest 1995;108(1):153-6. [CFGD REGISTER: DT13] - PubMed
Geller 1998 {published data only}
-
- Geller D. Aerosolized dornase alpha in cystic fibrosis: is there a role in the management of patients with early obstructive lung disease? Pediatric Pulmonology 1997;24 (Suppl 2):155-8. [CFGD REGISTER: DT8c] - PubMed
-
- Geller DE, Eigen H, Fiel S, Lamarre A, Konstan M, Johnson C. Aerosolized dornase alpha in cystic fibrosis: smaller particle size may improve outcome in patients with mild obstructive lung disease. Israel Journal of Medical Sciences 1996;32 Suppl:S35. [CFGD REGISTER: DT8a]
-
- Geller DE, Eigen H, Fiel SB, Clark A, Lamarre AP, Johnson CA, et al. Effect of smaller droplet size of dornase alpha on lung function in mild cystic fibrosis. Pediatric Pulmonology 1998;25 (Suppl 2):83-7. [CFGD REGISTER: DT8b] - PubMed
Geller 2003 {published data only}
-
- Geller DE, Rosenfeld M, Waltz DA, Wilmott RW. Efficiency of pulmonary administration of tobramycin solution for inhalation in cystic fibrosis using an improved drug delivery system. Chest 2003;123(1):28-36. [CFGD REGISTER: DT27b] - PubMed
-
- Wilmott RW, Chatfield B, Dyson M, Geller D, Milgram L, Noone PG, et al. Aerodose improves TOBI delivery in cystic fibrosis. Pediatric Pulmonology 2001;32 (Suppl 22):292. [CFGD REGISTER: DT27a]
Govoni 2013 {published data only}
-
- Govoni M , Poli G , Acerbi D , Santoro D , Cicirello H , Annoni O, et al. Pharmacokinetic and tolerability profiles of tobramycin nebuliser solution300 mg/4 ml administered by PARI eFlow® rapid and PARI LC Plus® nebulisersin cystic fibrosis patients. Pulmonary Pharmacology & Therapeutics 2013;26:249-55. - PubMed
-
- Govoni M, Poli G, Acerbi D, Santoro D, Cicirello H, Annoni O, Ruzicka J. Pharmacokinetic and tolerability profiles of tobramycin nebuliser solution 300 mg/4 ml administered by PARI eFlow((R)) rapid and PARI LC Plus((R)) nebulisers in cystic fibrosis patients. Pulmonary Pharmacology & Therapeutics 2013;26(2):249-55. [CFGD REGISTER: DT46b] - PubMed
-
- Govoni M, Poli G, Cicirello H, Santoro D, Acerbi D, Ruzicka J. Pharmacokinetics of tobramycin nebulizer solution (300mg/4ml) administered by Pari e-Flow rapid vs Pari LC plus nebulizer in patients with cystic fibrosis and Pseudomonas aeruginosa infection. European Respiratory Journal 2012;40 (Suppl 56):606s. [CFGD REGISTER: DT46c]
-
- Govoni M, Poli G, Cicirello H, Santoro D, Acerbi D, Ruzièka J. Pharmacokinetics of tobramycin nebulizer solution (Bramitob®) administered by Pari e-Flow Rapid vs Pari LC Plus nebulizer in patients with cystic fibrosis and Pseudomonas aeruginosa infection. Journal of Cystic Fibrosis 2012;10 (Suppl 1):S129, Abstract no: 285. [CFGD REGISTER: DT46a]
-
- NCT01116089. Pharmacokinetic study of bramitob® administered for inhalation by PARI eFlow® vs PARI LC® PLUS nebulizer. clinicaltrials.gov/ct2/show/NCT01116089 (date first posted 04 May 2010). [CFGD REGISTER: DT46d]
Griese 2014 {published data only}
-
- Griese M, Eismann C, Borner G, Denk O, Schierholz JM, Keller M, et al. A pharmacokinetics and safety comparison of a highly concentrated tobramycin solution with TOBI. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2014;27(3):185-92. [CFGD REGISTERE: DT39b] - PubMed
-
- Griese M, Kappler M, Eismann C, Mazurek H, Denk O, Goedings P, et al. Pulmonary delivery of tobramycin by an investigational Eflow® electronic nebulizer saves 50% of the drug, and results in equivalent or improved tobramycin safety levels in plasma and sputum. Pediatric Pulmonology 2009;44 (Suppl 32):300, Abstract no: 252. [CFGD REGISTER: DT39a]
Haussermann 2016 {published data only}
-
- Haussermann S, Winnips C, Edelman J, Kappeler D, Herpich C, Ehlich H, et al. Lung deposition of alpha-proteinase inhibitor (human) (A-PI[H]) inhalation solution using two inhalation modes of the I-neb adaptive aerosol delivery (AAD) system in healthy subjects and subjects with cystic fibrosis. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2016;29(3):242-50. [CFGD REGISTER: DT56] - PubMed
Hubert 2009 {published data only}
-
- Hubert D, Leroy S, Dominique S, Kovarik J. Pharmacokinetic comparison of inhaled tobramycin (TOBI) via Pari eFlow rapid or Pari LC Plus nebulizers in cystic fibrosis. Journal of Cystic Fibrosis 2007;6 (Suppl 1):S12. [CFGD REGISTER: DT37a] - PubMed
-
- Hubert D, Leroy S, Nove-Josserand R, Murris-Espin M, Mely L, Dominique S, et al. Pharmacokinetics and safety of tobramycin administered by the PARI eFlow rapid nebulizer in cystic fibrosis. Journal of Cystic Fibrosis 2009;8(5):332-7. [CFGD REGISTER: DT37c] - PubMed
-
- Tavakkol A, Hubert D, Leroy S, Nove-Josserand R, Murris-Espin M, Mely L, et al. Tobramycin inhalation solution (TOBI) administered via Pari Eflow Rapid nebuliser may yield a higher therapeutic ratio vs Pari LC Plus jet nebuliser in cystic fibrosis patients. Pediatric Pulmonology 2007;42 (Suppl 30):289. [CFGD REGISTER: DT37b]
Kastelik 2002 {published data only}
-
- Kastelik JA, Aziz I, Wright GA, Davies M, Morice AH. Comparitive assessment of aerosol delivery in cystic fibrosis. Thorax 2000;55 (Suppl 3):A77. [CFGD REGISTER: DT18b]
-
- Kastelik JA, Davies M, Wright GA, Aziz I, Morice AH. Radiolabelled DTPA as a tool to study nebuliser performance: a comparison of two systems in cystic fibrosis and healthy volunteers. Pediatric Pulmonology 2000;30 (Suppl 20):247. [CFGD REGISTER: DT18a]
-
- Kastelik JA, Davies M, Wright GA, Aziz I, Morice AH. Radiolabelled DTPA as a tool to study nebuliser performance: a comparison of two systems in cystic fibrosis and healthy volunteers. Pediatric Pulmonology 2000;Suppl 20:247. [CF REGISTER: DT18a]
-
- Kastelik JA, Wright GA, Aziz A, Davies M, Avery GR, Paddon AJ, et al. A widely available method for the assessment of aerosol delivery in cystic fibrosis. Pulmonology Pharmacology & Therapeutics 2002;15(6):513-9. [CFGD REGISTER: DT18c] - PubMed
-
- Kastelik JA, Wright GA, Aziz I, Davies M, Avery GR, Paddon AJ, et al. A widely available method for the assessment of aerosol delivery in cystic fibrosis. Pulmonary Pharmacology & Therapeutics 2002;15(6):513-9. [CF REGISTER: DT18c] - PubMed
Köhler 2003 {published data only}
-
- Köhler E, Sollich V, Schuster R, Hühnerbein. Lung deposition in CF patients using ultrasonic or jet nebulising. In: XIIIth International Cystic Fibrosis Conference; 2000 4-8 June, Stockholm, Sweden. 2000:144. [CFGD REGISTER: DT19a]
-
- Köhler E, Sollich V, Schuster R, Wonka V, Jorch G. Lung deposition following electronically breath controlled inhalation and manually triggered conventional inhalation in CF patients. Journal of Cystic Fibrosis 2004;3 (Suppl 1):S65. [CFGD REGISTER: DT19b] - PubMed
-
- Köhler E, Sollich V, Schuster-Wonka R, Hühnerbein J. Lung deposition in cystic fibrosis patients using an ultrasonic or a jet nebuliser. Journal of Aerosol Medicine 2003;16(1):37-46. [CFGD REGISTER: DT19c] - PubMed
Lenney 2011 {published data only}
-
- Lenney W, Edenborough F, Kho P, Kovarik JM. Lung deposition of inhaled tobramycin with eFlow rapid/LC plus jet nebuliser in healthy and cystic fibrosis subjects. Journal of Cystic Fibrosis 2011;10(1):9-14. [CFGD REGISTER: DT44] - PubMed
Marshall 1994 {published data only}
-
- Marshall L, Reinhardt C, Lundberg T, Francis P, Khafagi F. Comparison of therapeutic nebuliser performance in cystic fibrosis using Tc-99m DTPA aerosol. Australian and New Zealand Journal of Medicine 1992;22(6):726. [CFGD REGISTER: DT7b]
-
- Marshall LM, Francis P, Khafagi FA. Aerosol deposition in cystic fibrosis using an aerosol conservation device and a conventional jet nebuliser. Journal of Paediatrics and Child Health 1994;30(1):65-7. [CFGD REGISTER: DT7a] - PubMed
McCormack 2011 {published data only}
-
- McCormack P, McNamara PS, Southern KW. A randomised controlled trial of breathing modes for adaptive aerosol delivery in children with cystic fibrosis. Journal of Cystic Fibrosis 2011;10(5):343-9. [CFGD REGISTER: DT45b] - PubMed
-
- McCormack P, McNamara PS, Southern KW. Open-label study: a comparison of breathing modes for adaptive aerosol delivery in children with cystic fibrosis. Journal of Cystic Fibrosis 2011;10 (Suppl 1):S54, Abstract no: 214. [CFGD REGISTER: DT45a] - PubMed
-
- McCormack P, Southern KW, McNamara PS. New nebulizer technology to monitor adherence and nebulizer performance in Cystic Fibrosis. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2012;25(6):307-9. [CFGD REGISTER: DT45c] - PubMed
NCT00399945 {published data only}
-
- NCT00399945. Tobramycin inhalation solution administered by eFlow rapid nebulizer: scintigraphy study. clinicaltrials.gov/ct2/show/NCT00399945 (date first posted 15 Nov 2006). [CFGD REGISTER: DT63]
-
- Novartis. CTBM100B 2202 - study results. www.novctrd.com/#/product?type=clinicalM&medicalConditionId=126&... (accessed 31 August 2022).
NCT00420836 {published data only}
-
- CTBM100B2201. www.novctrd.com/#/product?type=clinicalM&medicalConditionId=126&... (accessed 31 August 2022).
-
- NCT00420836. Tobramycin administered by eFlow rapid nebulizer: pharmacokinetic study. clinicaltrials.gov/ct2/show/NCT00420836 (date first posted 11 Jan 2007). [CFGD REGISTER: DT66]
Newman 1988 {published data only}
Sands 2014 {published data only}
-
- Sands D, Sapiejka E, Gaszczyk G, Mazurek H, for the T100 Study Group. Comparison of two tobramycin nebuliser solutions: Pharmokinetic, efficacy and safety profiles of T100 and TNS. Journal of Cystic Fibrosis 2014;13(6):653-60. [CFGD REGISTER: PI263c] - PubMed
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. A randomised comparison of two tobramycin formulations in cystic fibrosis (CF) patients with chronic pseudomonas aeruginosa (PA) infection for pharmacokinetic and therapeutic equivalence. Journal of Cystic Fibrosis 2013;12 (Suppl 1):S66, Abstract no: 69. [CFGD REGISTER: PI263a]
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. Use of an electronic monitoring system to generate objective information on patients' adherence to taking treatments of a novel inhaled tobramycin solution. European Respiratory Journal 2013;42:P1188, Abstract no: 1497. [CFGD REGISTER: PI263d]
-
- Sands D, Sapiejka E, Mazurek H, Gaszczyk G. Use of an electronic monitoring system to generate objective information on patients' adherence to taking treatments of a novel tobramycin solution (VANTOBRA). Journal of Cystic Fibrosis 2013;12 (Suppl 1):S66, Abstract no: 70. [CFGD REGISTER: PI263b]
Sawicki 2015 {published data only}
-
- Konstan MW, Chou W, Trzaskoma B, Xu Y. A study to evaluate the comparable efficacy and safety of Pulmozyme® delivered by the Erapid™ nebulizer system. Pediatric Pulmonology 2013;48 (Suppl 36):452, Abstract no: 668. [CFGD REGISTER: BD219c]
-
- NCT01712334. A study of the comparable efficacy and safety of pulmozyme (dornase alfa) delivered by the erapid nebulizer system in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT01712334 (date first posted 23 Oct 2012). [CFGD REGISTER: BD219e]
-
- Sawicki GS, Chou W, Raimundo K, Trzaskoma B, Konstan MW. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14(6):777-83. [CFGD REGISTER: BD219d] - PubMed
-
- Sawicki GS, Konstan M, Chou W, Trzaskoma B, Raimundo K. Impact of dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system on cystic fibrosis-related quality of life - results from the IMPART Study. Pediatric Pulmonology 2014;49 (Suppl 38):445-6, Abstract no: 621. [CFGD REGISTER: BD219a]
-
- Sawicki GS, Konston M, Chou W, Trzaskoma B, Raimundo K. Treatment preferences and satisfaction with dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system to patients with cystic fibrosis - results from the IMPART Study. Pediatric Pulmonology 2014;49 (Suppl 38):444, Abstract no: 617. [CFGD REGISTER: BD219b]
Shah 1997 {published data only}
-
- Shah PL, Scott SF, Geddes DM, Conway S, Carr S, Wallis C, et al. An evaluation of two aerosol delivery systems for rhDNase [abstract]. Israel Journal of Medical Sciences 1996;32 Suppl:S222. [CFGD REGISTER: DT11a] - PubMed
-
- Shah PL, Scott SF, Geddes DM, Conway S, Watson A, Nazir T, et al. An evaluation of two aerosol delivery systems for rhDNase. European Respiratory Journal 1997;10(6):1261-6. [CFGD REGISTER: DT11b] - PubMed
Thomas 1991 {published data only}
van Velzen 2015 {published data only}
-
- Velzen AJ, Shahbabai P, Uges JW, Touw DJ, Heijermann HG 2013. Influence of inhalation mode on aerosol lung deposition in patients with cystic fibrosis PART 1: pharmacokinetic data as representative of lung deposition. Journal of Cystic Fibrosis 2013;12(Suppl 1):S98. [ABSTRACT NO.: 197] [CFGD REGISTER: DT51b]
-
- Velzen AJ, Uges JW, Le Brun PP, Shahbabai P, Touw DJ, Heijerman HG. The influence of breathing mode on tobramycin serum levels using the I-neb AAD system in adults with cystic fibrosis. Journal of Cystic Fibrosis 2015;14(6):748-54. [CFGD REGISTER: DT51c] - PubMed
-
- Velzen AJ, Uges JW, Le Brun PP, Shahbabai P, Touw DJ, Heijerman HG. Influence of breathing pattern on pulmonary aerosol deposition in patients with cystic fibrosis (CF): a pharmacokinetic approach. Journal of Cystic Fibrosis 2014;13(Suppl 2):S30. [ABSTRACT NO.: WS14.5] [CFGD REGISTER: DT51a]
van Velzen 2016 {published data only}
-
- Bos AC, Velzen AJ, Touw DJ, Tiddens H, Heijerman HG, Janssens HM. Pharmacokinetics and tolerability in cystic fibrosis of double dose nebulized tobramycin using controlled and conventional inhalation [Pharmacokinetics and tolerability in cystic fibrosis of double dose nebulized tobramycin using controlled and conventional inhalation]. Pediatric Pulmonology 2015;50 Suppl 41:326. [ABSTRACT NO.: 357] [CFGD REGISTER: DT53b] - PubMed
-
- EUCTR2013-004488-30-NL. Targeting antibiotics to Pseudomonas aeruginosa in small airways (TAPAS) study in patients with cystic fibrosis: pharmacokinetics (PK) [Targeting Antibiotics to Pseudomonas Aeruginosa in Small airways (TAPAS) study in patients with cystic fibrosis: pharmacokinetics (PK) - TAPAS-PK study in patients with CF]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-004488-30-NL (first registered 11 December 2013). [CF REGISTER: DT53f]
-
- EUCTR2014-001401-41-NL. Once daily deep inhalation of tobramycin with smart nebulizer more effective to treat small airways disease in cystic fibrosis? [Targeting Antibiotics to Pseudomonas Aeruginosa in Small airways (TAPAS) study in patients with cystic fibrosis - TAPAS study in patients with CF]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-001401-41-NL (date first registered 15 May 2014). [CF REGISTER: DT71a]
-
- NTR4525. Targeting antibiotics to pseudomonas aeruginosa in small airways (TAPAS) study in patients with cystic fibrosis: pharmacokinetics (PK). trialsearch.who.int/Trial2.aspx?TrialID=NTR4525 (date first registered 16 Apr 2014). [CFGD REGISTER: DT53e]
-
- NTR5211. TAPAS study in patients with CF [Targeting Antibiotics to Pseudomonas Aeruginosa in Small airways (TAPAS) study in patients with cystic fibrosis - TAPAS study in patients with CF]. trialsearch.who.int/Trial2.aspx?TrialID=NTR5211 (date first registered 4 May 2015). [CF REGISTER: DT71b]
van Velzen 2019 {published data only}
-
- EUCTR2012-002503-17-NL. Tobramycin nebulisation with I-neb (TONI) study in children with cystic fibrosis: pharmacokinetics and safety. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-002503-17-NL (date first registered 14 Aug 2012). [CFGD REGISTER: DT52c]
-
- NTR4216. Tobramycin nebulisation with I-neb (TONI) study in children with cystic fibrosis: pharmacokinetics and safety. trialsearch.who.int/Trial2.aspx?TrialID=NTR4216 (date first registered 21 Oct 2013). [CFGD REGISTER: DT52d]
-
- Velzen AJ, Uges JW, Arets HG, Nuijsink M, Wiel-Kooij EC, Pullens B, et al. Tobramycin nebulization with I-neb®in children with cystic fibrosis (TONI study): pharmacokinetics and safety. Journal of Cystic Fibrosis 2015;14 (Suppl 1):S49, Abstract no: ePS04.7. [CFGD REGISTER: DT52a]
-
- Velzen AJ, Uges JW, Heijerman HG, Arets BG, Nuijsink M, Wiel-Kooij EC, et al. Pharmacokinetics and safety of tobramycin nebulization with the I-neb and PARI-LC Plus in children with cystic fibrosis: a randomized, crossover study. British Journal of Clinical Pharmacology 2019;85(9):1984-93. [CFGD REGISTER: DT61] - PMC - PubMed
-
- Velzen AJ, Uges JW, Heijerman HG, Arets HG, Nuijsink M, Wiel-Kooij EC, et al. Tobramycin nebulization with the I-neb®in children with cystic fibrosis (TONI study): pharmacokinetics and safety. Journal of Cystic Fibrosis 2017;16 (Suppl 1):S25-S26. [CFGD REGISTER: DT52b]
Westerman 2008 {published data only}
-
- Westerman EM, Boer AH, Brun PP, Roldaan AC, Frijlink HW, Heijerman HG. Aerosolization of tobramycin (TOBI) with the PARI LC plus reusable nebulizer: which compressor to use? Comparison of the CR60 to the portaneb compressor. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2008;21(3):269-80. [CFGD REGISTER: DT38b] - PubMed
-
- Westerman EM, Touw DJ, Heijerman HG. Portaneb or CR60? Inhalation of tobramycin (TOBI) with the Pari LC Plus nebuliser: an in-vivo pilot study. Journal of Cystic Fibrosis 2007;6 (Suppl 1):S39. [CFGD REGISTER: DT38a]
References to studies excluded from this review
Amelina 2021 {published data only}
-
- Amelina EL, Krasovskiy SA, Abdulganieva DI, Asherova IK, Zil'ber IE, Trishina SV, et al. Efficacy and safety of the biosimilar medicinal product Tigerase® (dornase alfa) in long-term symptomatic treatment of patients with cystic fibrosis: results of a phase III clinical trial. Pulmonologiya 2019;29(6):695-706. [CFGD REGISTER: BD278a]
-
- Amelina EL, Krasovsky SA, Akhtyamova-Givirovskaya NE, Kashirskaya NY, Abdulganieva DI, Asherova IK, et al. Comparison of biosimilar Tigerase and Pulmozyme in long-term symptomatic therapy of patients with cystic fibrosis and severe pulmonary impairment (subgroup analysis of a Phase III randomized open-label clinical trial (NCT04468100)). PLOS One 2021;16(12):e0261410. [CF REGISTER: BD278b] [DOI: 10.1371/journal.pone.0261410] - DOI - PMC - PubMed
Coldham 2004 {published data only}
-
- Coldham CM, Mulrennan SA, Wright G, Davies T, Seymour JD, Morice AH. A pilot study to investigate the lung deposition of aerosol nebulised solutions using two different nebuliser systems in to the lungs of cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2004;3(Suppl 1):S65. [CFGD REGISTER: DT29]
Conway 1993 {published data only}
-
- Conway SP, Watson A, Pond MN, Bayston S, Booth R, Ghonheim A. Sputum gentamicin (G) levels after delivery by rotahaler and nebuliser. Pediatric Pulmonology 1993;16 (Suppl 9):175. [CFGD REGISTER: DT9a]
-
- Watson A, Pond M, Bayston S, Booth R, Chonheim A, Conway SP. Sputum gentamicin (G) levels after delivery by rotahaler and nebuliser. In: 18th European Cystic Fibrosis Conference; 1993 May 21-26; Madrid, Spain. 1993:W2.1. [CFGD REGISTER: DT9b]
Crowther Labiris 1999 {published data only}
-
- Crowther Labris NR, Holbrook AM, Chrystyn H, Macleod SM, Newhouse MT. Dry powder versus intravenous and nebulized gentamycin in cystic fibrosis and bronchiectasis. A pilot study. American Journal of Respiratory and Critical Care Medicine 1999;160(5):1711-6. [CFGD REGISTER: DT17] - PubMed
Dentice 2009 {published data only}
-
- Dentice RL, Elkins MR, Bye PT. A randomised, cross-over trial of upright sitting versus alternate side lying during nebulised delivery of medication in cystic fibrosis. Journal of Cystic Fibrosis 2009;8 (Suppl 2):S74, Abstract no: 298. [CFGD REGISTER: DT49a]
-
- Dentice RL, Elkins MR, Bye PT. Does inhalation in side lying influence the delivery rate of nebulised medications in people with cystic fibrosis? Pediatric Pulmonology 2009;44 (Suppl 32):369, Abstract no: 446. [CFGD REGISTER: DT49b]
Dentice 2010 {published data only}
-
- Dentice RL, Elkins MR, Verschuer J, Eberl S, Bye PT. Does body position during inhalation of a nebulised aerosol influence the pattern of deposition in adults with and without cystic fibrosis. Journal of Cystic Fibrosis 2010;9 Suppl 1:S74, Abstract no: 286. [CFGD REGISTER: DT50]
Dentice 2019 {published data only}
-
- Dentice RL, Elkins MR, Verschuer J, Eberl S, Dwyer G, Bye PT. Side lying during nebulisation can significantly improve apical deposition in healthy adults and adults with mild cystic fibrosis lung disease: a randomised crossover trial. BMC Pulmonary Medicine 2019;19(1):128. [CFGD REGISTER: DT57b] - PMC - PubMed
Dolovich 2005 {published data only}
-
- Dolovich M, Eng P, Rhem R, Guo X, Labris R, Thabane L. Does particle size influence distribution of inhaled therapy in the presence of airways obstruction: a 3D PET study of the lung in cystic fibrosis. In: American Thoracic Society International Conference; 2005 May 20-25, San Diego, USA. 2005:A377. [CFGD REGISTER: DT31]
Donn 2016 {published data only}
-
- Donn KH, Ditcham W, Clements B, Devadason S, Murdzoska J, Busick D, et al. Pulmonary aerosol deposition in children with cystic fibrosis following transnasal delivery of hypertonic saline. Pediatric Pulmonology 2016;51 (Suppl 45):368. [CFGD REGISTER: DT40]
EUCTR2005‐004103‐10‐DE {published data only}
-
- EUCTR2005-004103-10-DE. Randomized, open labeled, multi center, active controlled, parallel 28 days safety and bioavailability study of Tobramycin 100 PARI nebulized with eFlow® versus TOBI® nebulized with PARI LC PLUS in cystic fibrosis patients with Pseudomonas Aeruginosa infections. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-004103-10-DE (date first registered 30 Jan 2006). [CFGD REGISTER: DT64]
EUCTR2007‐000959‐33‐DE {published data only}
-
- EUCTR2007-000959-33-DE. Randomized, open labelled, cross over deposition study of Tobramycin 100 PARI nebulized with eFlow® versus TOBI® nebulized with PARI LC PLUS® in subjects with CF. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-000959-33-DE (date first registered 04 Apr 2007). [CFGD REGISTER: DT65]
EUCTR2007‐005346‐20‐GB {published data only}
-
- EUCTR2007-005346-20-GB. Does nebulised tobramycin (TOBI) via e-flow delivery systems cause a raised peak serum tobramycin level in children with Cystic Fibrosis? - TOBICF. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-005346-20-GB (date first registered 28 Nov 2007). [CFGD REGISTER: DT67]
EUCTR2009‐013660‐39‐FR {published data only}
-
- EUCTR2009-013660-39-FR. Etude pharmacocinetique de l’equivalence de la biodisponibilite entre Nebcinal® 150mg/3ml administre par Aeroneb® Idehaler® et Tobi® 300mg/5ml administre par Pari LC Plus ®. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-013660-39-FR (date first registered 27 Nov 2009). [CFGD REGISTER: DT68]
EUCTR2010‐023235‐41‐PL {published data only}
-
- EUCTR2010-023235-41-PL. A comparative, randomised, two period, multi-center, cross-over 14 weeks bioequivalence study of Tobramycin PARI (T100) versus TOBI® (Novartis) in cystic fibrosis patients with bronchopulmonary chronic Pseudomonas aeruginosa infection - T-100 BE. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2010-023235-41-PL (date first registered 28 Jan 2011). [CFGD REGISTER: DT76]
EUCTR2010‐023533‐34‐FR {published data only}
-
- EUCTR2010-023533-34-FR. Etude pharmacocinetique de l’equivalence de la biodisponibilite entre Nebcinal® 150mg/3ml administre par Aeroneb® Idehaler® et Tobi® 300mg/5ml administre par Pari LC Plus ® /Pulmoaid ® chez des patients atteints de mucoviscidose. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2010-023533-34-FR (date first registered 14 Dec 2010). [CFGD REGISTER: DT70]
Eyns 2015 {published data only}
-
- Eyns H, Willekens J, Gaspar V, Opdekamp C, Van Hove O, Galasso C, et al. Use of the AKITA JET for inhalation treatment in cystic fibrosis - part I: clinical outcomes. Journal of Cystic Fibrosis 2015;14 (Suppl 1):S15, Abstract no: WS08.2/1. [CFGD REGISTER: DT54a]
-
- Eyns H, Willekens J, Opdekamp C, Van Hove O, Gaspar V, Galasso C, et al. Use of the AKITA JET for inhalation treatment in cystic fibrosis - part II: patients' satisfaction. Journal of Cystic Fibrosis 2015;14 (Suppl 1):S15, Abstract no: WS08.2/2. [CFGD REGISTER: DT54b]
Faroux 2000 {published data only}
-
- Fauroux B, Itti E, Pigeot J, Isabey D, Meignan M, Ferry G, et al. Optimization of aerosol deposition by pressure support in children with cystic fibrosis: an experimental and clinical study. American Journal of Respiratory and Critical Care Medicine 2000;162(6):2265-71. [CFGD REGISTER: DT21a] - PubMed
-
- Itti E, Fauroux B, Pigeot J, Isabey D, Clement A, Evangelista E, et al. Quantitative lung perfusion scan as a predictor of aerosol distribution heterogeneity and disease severity in children with cystic fibrosis. Nuclear Medicine Communications 2004;25(6):563-9. [CFGD REGISTER: DT21b] - PubMed
Greenwood 2017 {published data only}
-
- Greenwood J, Schwarz C, Sommerwerck U, Nash EF, Tamm M, Cao W, et al. Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: a crossover study in cystic fibrosis patients with pulmonary Pseudomonas aeruginosa infection. Therapeutic Advances in Respiratory Disease 2017;11(17):249-60. [CFGD REGISTER: DT62b] - PMC - PubMed
-
- Greenwood J, Schwarz C, Sommerwerck U, Nash EF, Tamm M, Cao W, et al. Microbial contamination profile of tobi podhaler™ versus nebulizers used in cystic fibrosis patients with chronic pseudomonas aeruginosa infection: a real-world study. Pediatric Pulmonology 2016;51 (Suppl):315. [CFGD REGISTER: DT62a]
Hung 1995 {published data only}
-
- Hung JC, Spring M, Hambleton G, Super M. A preliminary study on the delivery of gentamycin dry powder to the lung of children with cystic fibrosis. In: 20th European Cystic Fibrosis Conference; 1995 June 18-21; Brussels, Belgium. 1995:P53. [CFGD REGISTER: DT10]
Jirasek 2022 {published data only}
-
- Jirasek M, Homola L, Pleskova J, Gracova Z, Benesova K, Hodkova P, Koucky V. “CF Hero” application as a motivational and therapeutic tool for kids and teenagers with cystic fibrosis. Journal of Cystic Fibrosis 2022;21 Suppl 1:S130. [CF REGISTER: MH206] [DOI: 10.1016/S1569-1993(22)00556-2] - DOI
Johnson 2006 {published data only}
-
- Johnson JC, Waldrep JC, Dhand R. Aerosol delivery of recombinant human DNase 1. Comparison of a vibrating mesh nebulizer with a jet nebulizer. In: American Thoracic Society International Conference; 2006 May 19-24; California, USA. 2006:A82.
Kovaleva 2001 {published data only}
-
- Kovaleva LF, Gembitskaya TE, Zaremba IA. The optimization of the therapy in cystic fibrosis (CF) patients using nebuliser. In: 24th European Cystic Fibrosis Conference; 2001 June 6- 9; Vienna, Austria. 2001:P86. [CFGD REGISTER: DT24]
Laube 2000 {published data only}
-
- Laube BL, Jashnani R, Dalby RN, Zeitlin PL. Targeting aerosol deposition in patients with cystic fibrosis: effects of alterations in particle size and inspiratory flow rate. Chest 2000;118(4):1069-76. [CFGD REGISTER: DT20] - PubMed
Liew 2011 {published data only}
-
- Liew LK, Gangelll CL, Schultz EN, Minocchieri1 S, Burchell JT, Devadason SG. Optimal delivery of cystic fibrosis (CF) therapy from commonly recommended nebulisers. Respirology 2011;16 (Suppl 1):P55. [CFGD REGISTER: DT69]
Lumley 2022 {published data only}
-
- Lumley E, Drabble SJ, Scott A, Wildman MJ, O'Cathain A. Objective nebuliser adherence data AS "proof" of adherence in the management of cystic fibrosis: a qualitative interview study. Patient Preference and Adherence 2022;16:771-80. [CF REGISTER: MH205g] [DOI: 10.2147/PPA.S353434] - DOI - PMC - PubMed
Mallol 1996 {published data only}
-
- Mallol J, Rattray S, Walker G, Cook D, Robertson CF. Aerosol deposition in infants with cystic fibrosis. Pediatric Pulmonology 1996;21(5):276-81. [CFGD REGISTER: DT14] - PubMed
Mallol 1997 {published data only}
-
- Mallol J, Robertson CF, Cook D, Kaymakci B. Nebulized gentamicin in children with cystic fibrosis: Enhancing antibiotic lung deposition by increasing flow rate and fill volume. Journal of Aerosol Medicine 1997;10(4):331-40. [CFGD REGISTER: DT16]
Militz 2008 {published data only}
-
- Militz S, Feilcke M, Schroter C, Kraxner A, Eismann C, Griese M, et al. e-Flow rapid®: improved lung function and patient satisfaction with more volume delivered to the lungs during inhalation therapy in cystic fibrosis. Journal of Cystic Fibrosis 2008;7 (Suppl 2):S67. [CFGD REGISTER: DT47]
Moss 2005 {published data only}
-
- Moss RB, Mayer-Hamblett N, Daines C, Hale K, Gibson RL, Anderson P, et al. Randomised, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatric Pulmonology 2005;39(3):209-18. [CFGD REGISTER: IB92] - PubMed
Mulrennan 2004 {published data only}
-
- Mulrennan SA, Moon TM. Prodose nebuliser usage compared with usual nebuliser in cystic fibrosis patients. In: American Thoracic Society International Conference; 2004 May 21- 26; Florida, USA. 2004:A391.
NCT01288170 {published data only}
-
- NCT01288170. Pharmacokinetic bioequivalence study of nebcinal® 150mg/3ml administered by aeroneb® idehaler® versus tobi® 300mg/5ml administered by pari LC plus®. clinicaltrials.gov/ct2/show/NCT01288170 (date first posted 02 Feb 2011). [CFGD REGISTER: DT75]
NCT01337219 {published data only}
-
- NCT01337219. Pharmacokinetic bioequivalence study of nebcinal® 150mg/3ml administered by aeroneb® idehaler® versus tobi® 300 mg/5 ml administered by pari LC plus® /pulmoaid® in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT01337219 (date first posted 18 Apr 2011). [CFGD REGISTER: DT74]
NCT02538588 {published data only}
-
- NCT02538588. Comparison of amikacin lung delivery with two nebulizers. clinicaltrials.gov/ct2/show/NCT02538588 (date first posted 02 Sep 2015). [CFGD REGISTER: DT72]
Podolec 2001 {published data only}
-
- Podolec Z. Pneumodosimeter - the application of BCTS method (bronchial control treatment systems) to breath-controlled application of medicine aerosols in water solutions to persons suffering from mucoviscidosis. In: 24th European Cystic Fibrosis Conference; 2001 June 6-9; Vienna, Austria. 2001:P98. [CFGD REGISTER: DT25]
Potter 2008 {published data only}
-
- Potter RW, Hurren TJ, Nickerson C, Hatley RH. Comparison of the delivery characteristics of dornase alpha from the I-Neb AAD system and the sidestream et nebulizer. Pediatric Pulmonology 2008;43 (Suppl 31):361.
Su 2014 {published data only}
-
- Su S, Riccobene T, Scott C. Lung deposition of inhaled colistimethate sodium in cystic fibrosis patients. European Respiratory Journal 2014;44(Suppl 58):1975. [CFGD REGISTER: DT55]
Thee 2021 {published data only}
-
- DRKS00024642. Coaching and telemonitoring in patients with cystic fibrosis: connect CF. trialsearch.who.int/?TrialID=DRKS00024642 (date first registered 01 Mar 2021). [CFGD REGISTER: MH195a]
Vanlaethem 2008 {published data only}
-
- Van Ginderdeuren F, Vanlaethem S, Eyns H, De Schutter I, De Wachter E, Malfroot A. Influence of inhaled hypertonic saline (NaCl 6%) before or during autogenic drainage on sputum weight, oxygen saturation, heart frequency and dyspnea in cystic fibrosis patients. Journal of Cystic Fibrosis 2011;10 (Suppl 1):S62. [CFGD REGISTER: BD129b]
-
- Vanlaethem S, Van Ginderdeuren F, Eyns H, Malfroot A. Influence of inhaled hypertonic saline combined with airway clearance on SpO2, heart rate, dyspnoea and wet sputum weight in hospitalised CF patients. Journal of Cystic fibrosis 2008;7 (Suppl 2):S71. [CFGD REGISTER: BD129a]
Ward 2022 {published data only}
-
- Ward N, Ward B, Stiller K, Kenyon A, Holland AE. Development of a device to measure adherence and pressure characteristics of positive expiratory pressure therapies used by adults with cystic fibrosis. Physiotherapy Theory and Practice 2022;38(10):1469-77. [CF REGISTER: PE290b] [DOI: 10.1080/09593985.2020.1858465] - DOI - PubMed
-
- Ward N, Ward B, Stiller K, Kenyon A, Holland AE. Expiratory duration and pressure properties of commonly used airway clearance devices when used unsupervised by adults with cystic fibrosis. Pediatric Pulmonology 2019;54 (Suppl 2):382. [CFGD REGISTER: PE290a]
Wildman 2021 {published data only}
-
- Arden MA, Drabble S, O'Cathain A, Hutchings M, Wildman M. Adherence to medication in adults with Cystic Fibrosis: an investigation using objective adherence data and the Theoretical Domains Framework. British Journal of Health Psychology 2019;24(2):357-80. [CF REGISTER: MH53p] [DOI: 10.1111/bjhp.12357] - DOI - PMC - PubMed
-
- Arden MA, Drabble SJ, O’Cathain A, Hutchings M, Wildman M. ACtiF study: understanding adherence to nebuliser treatment in adults with cystic fibrosis using the Theoretical Domains Framework. Journal of Cystic Fibrosis 2016;15 Suppl(Suppl):S26. [DOI: 10.1016/S1569-1993(16)30151-5] [PMID: ] - DOI
-
- Arden MA, Hutchings M, Nightingale JA, Allenby M, Dewar J, Oliver C, et al. CFHealthHub: understanding psychosocial differences between high, medium and low adherers to nebuliser treatment: an exploratory analysis of data from CFHealthHub two centre pilot trial. Journal of Cystic Fibrosis 2017;16 (Suppl 1):S60. [CFGD REGISTER: MH53d]
-
- Choyce J, Dawson S, Rashid R, Whitehouse J, Patel N. The introduction of an adherence support clinic in a large UK adult cystic fibrosis centre. Journal of Cystic Fibrosis 2022;21:S130-1. [CF REGISTER: MH53v] [DOI: 10.1016/S1569-1993(22)00558-6] - DOI
Wilson 2015 {published data only}
-
- Wilson P, Lambert C, Nwokoro C. Safety of nebulised hypertonic saline via Pari eFlow with Pari positive expiratory pressure (PEP) device in circuit for paediatric cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14 (Suppl 1):S101, Abstract no. 170. [CFGD REGISTER: DT73]
Additional references
Cantin 1995
-
- Cantin A. Cystic Fibrosis lung inflammation: early, sustained and severe. American Journal of Respiratory and Critical Care Medicine 1995;151(4):939-41. - PubMed
CF Storm
-
- Southern KW, Davies G. A randomised open label trial to assess change in respiratory function for people with cystic fibrosis (pwCF) established on triple combination therapy (Kaftrio ) after rationalisation of nebulised muco-active therapies (the CF STORM trial). fundingawards.nihr.ac.uk/award/NIHR131889 (start date February 2021). [DOI: 10.1186/ISRCTN14081521] - DOI
CF Trust 2020
-
- CF Trust. Nebulised/inhaled medications for people with cystic fibrosis overview. Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis (Fourth Edition) 2020:211-221.
CFF 2020
-
- Cystic Fibrosis Foundation. National Patient Registry. Annual Data Report (Bethesda, Maryland). National Patient Registry 2020.
Collins 2009
Davies 2009
-
- Davies J, Alton E. Monitoring respiratory disease severity in cystic fibrosis. Respiratory Care 2009;54(5):606-17. - PubMed
Davis 2006
-
- Davis PB. Davis PB. Cystic fibrosis since 1938. American Journal of Respiratory and Critical Care Medicine March 2006;173(5):475-82. [PMID: doi: 10.1164/rccm.200505-840OE. Epub 2005 Aug 26. PMID: 16126935.] - PubMed
Dawson 2023
De Boeck 2020
-
- De Boeck, K. Cystic Fibrosis in the year 2020: A disease with a new face. Acta Paediatrica January 2020;109:893-9. [PMID: ] - PubMed
Denyer 2010
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
ERS 2001
-
- European Respiratory Society Task Force. European Respiratory Society Guidelines on the use of nebulizers. European Respiratory Journal 2001;18:228–42. - PubMed
Fischer 2009
Gee 2000
Halfhide 2005
Heijerman 2009
-
- Heijerman H, Westerman E, Conway S, Touw D, Döring G, for the consensus working group. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus. Journal of Cystic Fibrosis 2009;8(5):295–315. - PubMed
Henry 2003
-
- Henry B, Aussage P, Grosskopf C, Goehrs JM. Development of the Cystic Fibrosis Questionnaire (CFQ) for assessing quality of life in pediatric and adult patients. Quality of Life Research 2003;12(1):63-76. - PubMed
Higgenbottam 1997
-
- Higgenbottam T. Key issues in nebulized drug delivery to adults. European Respiratory Review 1997;7(51):378-9.
Higgins 2003
Higgins 2017
-
- Higgins JP Altman DG, Sterne JA on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook. Available from www.training.cochrane.org/handbook.
Hindle 1992
Hodson 2000
-
- Hodson M. Treatment of cystic fibrosis in the adult. Respiration 2000;67(6):595-607. - PubMed
Kesser 2009
-
- Kesser KC, Geller DE. New aerosol delivery devices for cystic fibrosis. Respiratory Care 2009;54(6):754-68. - PubMed
Konstan 1997
-
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatric Pulomonolgy 1997;24(2):137-42. - PubMed
NCT04378153
-
- NCT04378153. Impact of discontinuing chronic therapies in people with cystic fibrosis on highly effective CFTR modulator therapy (SIMPLIFY). clinicaltrials.gov/ct2/show/NCT04378153 (date first posted 7 May 2020).
NICE 2020
-
- NICE. CFHealthHub for managing cystic fibrosis during the COVID-19 pandemic. Medtech innovation briefing Medtech innovation briefing [MIB219] 2020.
Nissenbaum 2020
-
- Nissenbaum, C, Davies, G, Horsley, A & Davies, JC. Monitoring early stage lung disease in cystic fibrosis. Current Opinion in Pulmonary Medicine Nov 2020;26(6):671-6. [PMID: ] - PubMed
O'Callaghan 1997
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. - PMC - PubMed
RevMan 2011 [Computer program]
-
- Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
RevMan Web [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rosenfeld 2001
-
- Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, et al. Defining a pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 2001;139(3):359-65. - PubMed
Rosenstein 1998
-
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. Journal of Pediatrics 1998;132(4):589-95. [PMID: ] - PubMed
Sawicki 2009
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). The Cochrane Collaboration, 2022. Available from training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). The Cochrane Collaboration, 2022. Available from training.cochrane.org/handbook.
Scotet 2020
Smith 2023
Wildman 2022
References to other published versions of this review
Daniels 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

