Impact of Baseline Corticosteroid Use on the Efficacy and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Post Hoc Analysis of the Phase 3 Clinical Trial Programme
- PMID: 37942921
- PMCID: PMC11140624
- DOI: 10.1093/ecco-jcc/jjad190
Impact of Baseline Corticosteroid Use on the Efficacy and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Post Hoc Analysis of the Phase 3 Clinical Trial Programme
Abstract
Background and aims: This post hoc analysis assessed the efficacy and safety of upadacitinib in patients with moderately to severely active ulcerative colitis stratified by corticosteroid use from the ulcerative colitis Phase 3 clinical trial programme.
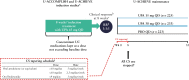
Methods: Patients were randomised [1:2] to 8 weeks' placebo or upadacitinib 45 mg once daily; Week 8 responders were re-randomised [1:1:1] to 52 weeks' placebo or upadacitinib 15 or 30 mg daily. Corticosteroid dose was kept stable during induction but tapered according to a protocol-defined schedule [or investigator discretion] during maintenance Weeks 0-8. Efficacy outcomes and exposure-adjusted, treatment-emergent adverse event [TEAE] rates were assessed for induction and maintenance stratified by corticosteroid use at induction baseline.
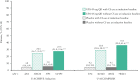
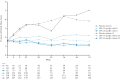
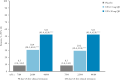
Results: Overall, 377/988 [38%] patients were receiving corticosteroids at induction baseline [placebo, n = 133; upadacitinib 45 mg, n = 244] and 252 [37%] of the 681 clinical responders who entered maintenance were on corticosteroids at induction baseline [n = 84 for each treatment]. Similar proportions of patients receiving upadacitinib achieved clinical remission per Adapted Mayo Score with and without baseline corticosteroids at Weeks 8 and 52. The total proportion of patients re-initiating corticosteroids was higher with placebo [24/84;29%] vs upadacitinib 15 mg [16/81; 20%)] and 30 mg [11/81; 14%]. During induction, patients receiving corticosteroids at baseline had higher rates of TEAEs, serious TEAEs, and serious infections vs those not receiving corticosteroids; however, TEAE rates were similar during maintenance after corticosteroid withdrawal.
Conclusions: Upadacitinib is an effective steroid-sparing treatment in patients with moderately to severely active ulcerative colitis. Clinicaltrials.gov identifiers: NCT02819635; NCT03653026.
Keywords: Clinical trials.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
TR received research/educational grants from AbbVie, Arena, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Janssen, LabGenius, MSD, Mylan, Novartis, Pfizer, Sandoz, Takeda, and UCB; speaker/consultation fees from AbbVie, Arena, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Janssen, LabGenius, MSD, Mylan, Novartis, Pfizer, Sandoz, Takeda, and UCB; and speaker and/or advisory board fees from AbbVie. YI received a research grant from Mitsubishi Tanabe Pharmaceutical; and speaker/consultation fees from AbbVie, Daiichi-Sankyo, EA Pharma, Janssen, and KISSEI Pharmaceutical. DTR received research funding from Takeda; has served as a consultant to AbbVie, Abgenomics, Allergan, Arena Pharmaceuticals, Biomica, BMS, Dizal, Ferring, Genentech/Roche, Janssen, Lilly, Mahana Therapeutics, Medtronic, Merck, Napo Pharmaceuticals, Pfizer, Prometheus Laboratories, Shire, Takeda, and Target PharmaSolutions; and is a co-founder of Cornerstones Health. TF-H is a former AbbVie employee and may hold AbbVie stock or stock options. RV, JL, CP, and EC are full-time employees of AbbVie and may hold AbbVie stock or stock options. LT received research funding from AbbVie Canada, Amgen Canada, Gilead Canada, Pfizer Canada, Roche Canada, Sandoz Canada, and Takeda Canada; and advisory board fees from AbbVie Canada, Amgen Canada, Janssen Canada, Merck Canada, Pfizer Canada, Roche Canada, Sandoz Canada, and Takeda Canada. EVL Jr received consulting fees from AbbVie, Alvotech, Amgen, Arena, Avalo Therapeutics, BMS, Boehringer Ingelheim, Calibr, Celgene, Celltrion, Eli Lilly, Fresenius Kabi, Genentech, Gilead, Gossamer Bio, GSK, Iota Biosciences, Iterative Scopes, Janssen, KSL Diagnostics, Ono Pharma, Morphic, Pfizer, Protagonist, Scipher Medicine, Sun Pharma, Surrozen, Takeda, and UCB; research support from AbbVie, BMS, Celgene, Genentech, Gilead, Gossamer Bio, Janssen, Pfizer, Receptos, Robarts Clinical Trials, Takeda, Theravance, and UCB; and is a shareholder of Exact Sciences.
Figures


References
-
- Ford AC, Bernstein CN, Khan KJ, et al.. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:590–9; quiz 600. - PubMed
-
- Lichtenstein GR, Rutgeerts P.. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2010;16:338–46. - PubMed
-
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol 2001;33:289–94. - PubMed

