High-Throughput Liquid Chromatographic Analysis Using a Segmented Flow Injector with a 1 s Cycle Time
- PMID: 37943345
- PMCID: PMC11027085
- DOI: 10.1021/acs.analchem.3c03719
High-Throughput Liquid Chromatographic Analysis Using a Segmented Flow Injector with a 1 s Cycle Time
Abstract
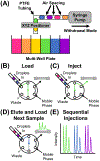
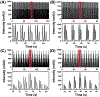
High-throughput screening (HTS) workflows are revolutionizing many fields, including drug discovery, reaction discovery and optimization, diagnostics, sensing, and enzyme engineering. Liquid chromatography (LC) is commonly deployed during HTS to reduce matrix effects, distinguish isomers, and preconcentrate prior to detection, but LC separation time often limits throughput. Although subsecond LC separations have been demonstrated, they are rarely utilized during HTS due to limitations associated with the speed of common autosamplers. In this work, these limits are overcome by utilizing droplet microfluidics for sample introduction. In the method, a train of samples segmented by air are continuously pumped into the inlet of an LC injection valve that is actuated once each sample fills the sample loop. Coupled with 2.1 mm diameter × 5 mm long columns packed with 2.7 μm superficially porous C18 particles operated at 5 mL/min, the injector enabled separation of 3 components at 1 s/sample and analysis of a 96-well plate in 1.6 min with <2% peak area relative standard deviation. Analyte-dependent carryover was minimized by including wash droplets composed of organic solvent in between sample droplets. High-throughput LC coupled with mass spectrometric detection using the segmented flow injector was applied to a screen of inhibitors of a cytochrome P450-catalyzed hydroxylation reaction. Measurements of the reaction substrate and product concentrations made using fast LC with the segmented flow injector correlated well with measurements made using a more conventional, 3 min LC method. These results demonstrate the potential for droplet microfluidics to be used for sample introduction during high-throughput LC analysis.
Figures


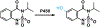
References
-
- Mennen SM; Alhambra C; Allen CL; Barberis M; Berritt S; Brandt TA; Campbell AD; Castañón J; Cherney AH; Christensen M; et al. Org. Process Res. Dev 2019, 23 (6), 1213–1242.
-
- Blay V; Tolani B; Ho SP; Arkin MR Drug Discovery Today 2020, 25 (10), 1807–1821. - PubMed
-
- Grainger R; Whibley S Org. Process Res. Dev 2021, 25 (3), 354–364.
-
- Santanilla AB; Regalado EL; Pereira T; Shevlin M; Bateman K; Campeau L-C; Schneeweis J; Berritt S; Shi Z-C; Nantermet P; et al. Science 2015, 347 (6217), 49–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

