Efficacy and Safety of Mazdutide in Chinese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial
- PMID: 37943529
- PMCID: PMC10733643
- DOI: 10.2337/dc23-1287
Efficacy and Safety of Mazdutide in Chinese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial
Abstract
Objective: We conducted a randomized, double-blind, placebo-controlled phase 2 trial to evaluate the efficacy and safety of mazdutide, a once-weekly glucagon-like peptide 1 and glucagon receptor dual agonist, in Chinese patients with type 2 diabetes.
Research design and methods: Adults with type 2 diabetes inadequately controlled with diet and exercise alone or with stable metformin (glycated hemoglobin A1c [HbA1c] 7.0-10.5% [53-91 mmol/mol]) were randomly assigned to receive 3 mg mazdutide (n = 51), 4.5 mg mazdutide (n = 49), 6 mg mazdutide (n = 49), 1.5 mg open-label dulaglutide (n = 50), or placebo (n = 51) subcutaneously for 20 weeks. The primary outcome was change in HbA1c from baseline to week 20.
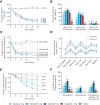
Results: Mean changes in HbA1c from baseline to week 20 ranged from -1.41% to -1.67% with mazdutide (-1.35% with dulaglutide and 0.03% with placebo; all P < 0.0001 vs. placebo). Mean percent changes in body weight from baseline to week 20 were dose dependent and up to -7.1% with mazdutide (-2.7% with dulaglutide and -1.4% with placebo). At week 20, participants receiving mazdutide were more likely to achieve HbA1c targets of <7.0% (53 mmol/mol) and ≤6.5% (48 mmol/mol) and body weight loss from baseline of ≥5% and ≥10% compared with placebo-treated participants. The most common adverse events with mazdutide included diarrhea (36%), decreased appetite (29%), nausea (23%), vomiting (14%), and hypoglycemia (10% [8% with placebo]).
Conclusions: In Chinese patients with type 2 diabetes, mazdutide dosed up to 6 mg was generally safe and demonstrated clinically meaningful HbA1c and body weight reductions.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

