FGF23 directly inhibits osteoprogenitor differentiation in Dmp1-knockout mice
- PMID: 37943605
- PMCID: PMC10807721
- DOI: 10.1172/jci.insight.156850
FGF23 directly inhibits osteoprogenitor differentiation in Dmp1-knockout mice
Abstract
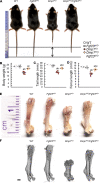
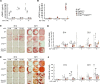
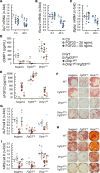
Fibroblast growth factor 23 (FGF23) is a phosphate-regulating (Pi-regulating) hormone produced by bone. Hereditary hypophosphatemic disorders are associated with FGF23 excess, impaired skeletal growth, and osteomalacia. Blocking FGF23 became an effective therapeutic strategy in X-linked hypophosphatemia, but testing remains limited in autosomal recessive hypophosphatemic rickets (ARHR). This study investigates the effects of Pi repletion and bone-specific deletion of Fgf23 on bone and mineral metabolism in the dentin matrix protein 1-knockout (Dmp1KO) mouse model of ARHR. At 12 weeks, Dmp1KO mice showed increased serum FGF23 and parathyroid hormone levels, hypophosphatemia, impaired growth, rickets, and osteomalacia. Six weeks of dietary Pi supplementation exacerbated FGF23 production, hyperparathyroidism, renal Pi excretion, and osteomalacia. In contrast, osteocyte-specific deletion of Fgf23 resulted in a partial correction of FGF23 excess, which was sufficient to fully restore serum Pi levels but only partially corrected the bone phenotype. In vitro, we show that FGF23 directly impaired osteoprogenitors' differentiation and that DMP1 deficiency contributed to impaired mineralization independent of FGF23 or Pi levels. In conclusion, FGF23-induced hypophosphatemia is only partially responsible for the bone defects observed in Dmp1KO mice. Our data suggest that combined DMP1 repletion and FGF23 blockade could effectively correct ARHR-associated mineral and bone disorders.
Keywords: Bone Biology; Bone disease; Genetic diseases; Metabolism; Osteoclast/osteoblast biology.
Figures







References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

