AUM302, a novel triple kinase PIM/PI3K/mTOR inhibitor, is a potent in vitro pancreatic cancer growth inhibitor
- PMID: 37943821
- PMCID: PMC10635512
- DOI: 10.1371/journal.pone.0294065
AUM302, a novel triple kinase PIM/PI3K/mTOR inhibitor, is a potent in vitro pancreatic cancer growth inhibitor
Abstract
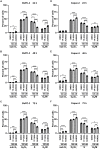
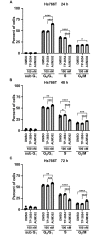
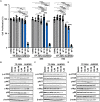
Pancreatic cancer is one of the leading causes of cancer deaths, with pancreatic ductal adenocarcinoma (PDAC) being the most common subtype. Advanced stage diagnosis of PDAC is common, causing limited treatment opportunities. Gemcitabine is a frequently used chemotherapeutic agent which can be used as a monotherapy or in combination. However, tumors often develop resistance to gemcitabine. Previous studies show that the proto-oncogene PIM kinases (PIM1 and PIM3) are upregulated in PDAC compared to matched normal tissue and are related to chemoresistance and PDAC cell growth. The PIM kinases are also involved in the PI3K/AKT/mTOR pathway to promote cell survival. In this study, we evaluate the effect of the novel multikinase PIM/PI3K/mTOR inhibitor, AUM302, and commercially available PIM inhibitor, TP-3654. Using five human PDAC cell lines, we found AUM302 to be a potent inhibitor of cell proliferation, cell viability, cell cycle progression, and phosphoprotein expression, while TP-3654 was less effective. Significantly, AUM302 had a strong impact on the viability of gemcitabine-resistant PDAC cells. Taken together, these results demonstrate that AUM302 exhibits antitumor activity in human PDAC cells and thus has the potential to be an effective drug for PDAC therapy.
Copyright: © 2023 Ingle et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. Epub 2010/04/28. doi: 10.1371/journal.pmed.1000267 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

