Aster-dependent nonvesicular transport facilitates dietary cholesterol uptake
- PMID: 37943936
- PMCID: PMC11073449
- DOI: 10.1126/science.adf0966
Aster-dependent nonvesicular transport facilitates dietary cholesterol uptake
Abstract
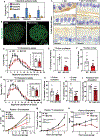
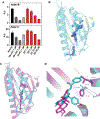
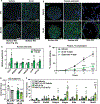
Intestinal absorption is an important contributor to systemic cholesterol homeostasis. Niemann-Pick C1 Like 1 (NPC1L1) assists in the initial step of dietary cholesterol uptake, but how cholesterol moves downstream of NPC1L1 is unknown. We show that Aster-B and Aster-C are critical for nonvesicular cholesterol movement in enterocytes. Loss of NPC1L1 diminishes accessible plasma membrane (PM) cholesterol and abolishes Aster recruitment to the intestinal brush border. Enterocytes lacking Asters accumulate PM cholesterol and show endoplasmic reticulum cholesterol depletion. Aster-deficient mice have impaired cholesterol absorption and are protected against diet-induced hypercholesterolemia. Finally, the Aster pathway can be targeted with a small-molecule inhibitor to manipulate cholesterol uptake. These findings identify the Aster pathway as a physiologically important and pharmacologically tractable node in dietary lipid absorption.
Conflict of interest statement
Figures




Update of
-
Aster-dependent non-vesicular transport facilitates dietary cholesterol uptake.bioRxiv [Preprint]. 2023 Jul 10:2023.07.07.548168. doi: 10.1101/2023.07.07.548168. bioRxiv. 2023. Update in: Science. 2023 Nov 10;382(6671):eadf0966. doi: 10.1126/science.adf0966. PMID: 37503112 Free PMC article. Updated. Preprint.
Comment in
-
New insights into dietary cholesterol absorption.Nat Rev Cardiol. 2024 Feb;21(2):70. doi: 10.1038/s41569-023-00975-y. Nat Rev Cardiol. 2024. PMID: 38040795 No abstract available.
References
-
- Buhman KK et al., Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nature Medicine 6, 1341–1347 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

