Blockade of IL-6R prevents preterm birth and adverse neonatal outcomes
- PMID: 37944273
- PMCID: PMC10665693
- DOI: 10.1016/j.ebiom.2023.104865
Blockade of IL-6R prevents preterm birth and adverse neonatal outcomes
Abstract
Background: Preterm birth preceded by spontaneous preterm labour often occurs in the clinical setting of sterile intra-amniotic inflammation (SIAI), a condition that currently lacks treatment.
Methods: Proteomic and scRNA-seq human data were analysed to evaluate the role of IL-6 and IL-1α in SIAI. A C57BL/6 murine model of SIAI-induced preterm birth was developed by the ultrasound-guided intra-amniotic injection of IL-1α. The blockade of IL-6R by using an aIL-6R was tested as prenatal treatment for preterm birth and adverse neonatal outcomes. QUEST-MRI evaluated brain oxidative stress in utero. Targeted transcriptomic profiling assessed maternal, foetal, and neonatal inflammation. Neonatal biometrics and neurodevelopment were tested. The neonatal gut immune-microbiome was evaluated using metagenomic sequencing and immunophenotyping.
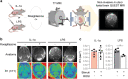
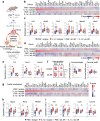
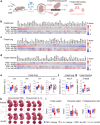
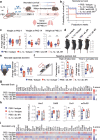
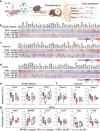
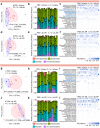
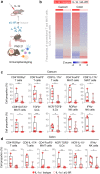
Findings: IL-6 plays a critical role in the human intra-amniotic inflammatory response, which is associated with elevated concentrations of the alarmin IL-1α. Intra-amniotic injection of IL-1α resembles SIAI, inducing preterm birth (7% vs. 50%, p = 0.03, Fisher's exact test) and neonatal mortality (18% vs. 56%, p = 0.02, Mann-Whitney U-test). QUEST-MRI revealed no foetal brain oxidative stress upon in utero IL-1α exposure (p > 0.05, mixed linear model). Prenatal treatment with aIL-6R abrogated IL-1α-induced preterm birth (50% vs. 7%, p = 0.03, Fisher's exact test) by dampening inflammatory processes associated with the common pathway of labour. Importantly, aIL-6R reduces neonatal mortality (56% vs. 22%, p = 0.03, Mann-Whitney U-test) by crossing from the mother to the amniotic cavity, dampening foetal organ inflammation and improving growth. Beneficial effects of prenatal IL-6R blockade carried over to neonatal life, improving survival, growth, neurodevelopment, and gut immune homeostasis.
Interpretation: IL-6R blockade can serve as a strategy to treat SIAI, preventing preterm birth and adverse neonatal outcomes.
Funding: NICHD/NIH/DHHS, Contract HHSN275201300006C. WSU Perinatal Initiative in Maternal, Perinatal and Child Health.
Keywords: Alarmin; IL-1α; Microbiome; Pregnancy; Prematurity; Sterile intra-amniotic inflammation.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Ream M.A., Lehwald L. Neurologic consequences of preterm birth. Curr Neurol Neurosci Rep. 2018;18(8):48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

