Alpha-lipoic acid does not improve olfactory training results in olfactory loss due to COVID-19: a double-blind randomized trial
- PMID: 37944311
- PMCID: PMC10665681
- DOI: 10.1016/j.bjorl.2023.101356
Alpha-lipoic acid does not improve olfactory training results in olfactory loss due to COVID-19: a double-blind randomized trial
Abstract
Objectives: Olfactory loss is a recognized long-term dysfunction after Coronavirus Disease 2019 (COVID-19) infection. This investigation aimed to assess the effect of alpha-lipoic acid as an adjuvant treatment of olfactory training on the improvement of smell loss in post-COVID-19 patients.
Methods: This randomized controlled trial included 128 adult outpatients who had persistent smell loss for more than 3-months after COVID-19 infection. The participants were randomly allocated into two groups: the intervention treatment group, which received alpha-lipoic acid associated to olfactory training, and comparison treatment group, which received placebo pills associated to olfactory training. The participants were followed-up for 12-weeks. Olfactory dysfunction was assessed in terms of Visual Analog Scale (VAS), and the Connecticut Chemosensory Clinical Research Center (CCCRC) test for the Brazilian population.
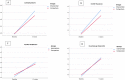
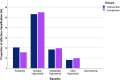
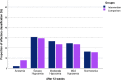
Results: A total of 100 participants completed the follow-up period and were analyzed in this study. Both groups have improved CCCRC score (p = 0.000), olfactory threshold (p = 0.000), identification score (p = 0.000) and VAS score (p = 0.000) after 12-weeks follow-up. No significant differences were determined between the intervention and comparison treatment groups in CCCRC score (p = 0.63), olfactory threshold (p = 0.50), identification score (p = 0.96) and VAS score (p = 0.97). In all these criteria, comparison treatment group went slightly worse. At the endpoint of the study, the frequency of anosmia reduced to 2% in the intervention treatment group and to 7.8% in the comparison treatment group. Also, 16.8% of the intervention group' subjects, and 15.7% of comparison treatment group's patients reached normosmia.
Conclusions: Overall, there was a strongly significant difference in olfactory function between baseline and endpoint for both groups. However, based on the lack of significant difference between the intervention treatment and the comparison treatment groups in terms of olfactory changes, our study appoints that the alpha-lipoic acid is not better than olfactory training alone to treat olfactory loss after COVID-19.
Level of evidence: Level 2.
Keywords: Alpha-lipoic acid; COVID-19; Olfactory dysfunction; Olfactory test; Post acute COVID-19 syndrome.
Copyright © 2023 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier España S.L.U. All rights reserved.
Figures
Similar articles
-
Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19.Laryngoscope. 2022 Nov;132(11):2209-2216. doi: 10.1002/lary.30353. Epub 2022 Aug 25. Laryngoscope. 2022. PMID: 36054369 Free PMC article. Clinical Trial.
-
Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction.Cochrane Database Syst Rev. 2021 Jul 22;7(7):CD013876. doi: 10.1002/14651858.CD013876.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Sep 5;9:CD013876. doi: 10.1002/14651858.CD013876.pub3. PMID: 34291813 Free PMC article. Updated.
-
Persistent COVID-19 parosmia and olfactory loss post olfactory training: randomized clinical trial comparing central and peripheral-acting therapeutics.Eur Arch Otorhinolaryngol. 2024 Jul;281(7):3671-3678. doi: 10.1007/s00405-024-08548-6. Epub 2024 Mar 16. Eur Arch Otorhinolaryngol. 2024. PMID: 38492007 Free PMC article. Clinical Trial.
-
Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: A randomized, double blind clinical trial.Int Immunopharmacol. 2021 Sep;98:107871. doi: 10.1016/j.intimp.2021.107871. Epub 2021 Jun 12. Int Immunopharmacol. 2021. PMID: 34147912 Free PMC article. Clinical Trial.
-
Efficacy of topical steroids for the treatment of olfactory disorders caused by COVID-19: A systematic review and meta-analysis.Clin Otolaryngol. 2022 Jul;47(4):509-515. doi: 10.1111/coa.13933. Epub 2022 Apr 6. Clin Otolaryngol. 2022. PMID: 35352483 Free PMC article.
Cited by
-
Treatment of COVID-19 Associated Olfactory Dysfunction: A Systematic Review.Curr Allergy Asthma Rep. 2024 Oct 31;25(1):2. doi: 10.1007/s11882-024-01182-6. Curr Allergy Asthma Rep. 2024. PMID: 39477832 Free PMC article.
-
A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings.Diagnostics (Basel). 2024 Feb 7;14(4):359. doi: 10.3390/diagnostics14040359. Diagnostics (Basel). 2024. PMID: 38396398 Free PMC article. Review.
-
Therapeutic options for the treatment of post-acute sequelae of COVID-19: a scoping review.BMC Infect Dis. 2025 May 22;25(1):731. doi: 10.1186/s12879-025-11131-x. BMC Infect Dis. 2025. PMID: 40405092 Free PMC article.
References
-
- Jonhs Hopkins’s COVID-19 Resource Center — tracking data until March 10th 2023. Website https://coronavirus.jhu.edu/map.html. [Accessed on 9 July 2023].
-
- Brazilian Health Ministry. Website https://covid.saude.gov.br/. [Accessed on 9 July 2023].
-
- Hummel T., Heilmann S., Huttenbriuk K.B. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002;112:2076–2080. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

