Sonochemistry dosimetries in seawater
- PMID: 37944338
- PMCID: PMC10654036
- DOI: 10.1016/j.ultsonch.2023.106647
Sonochemistry dosimetries in seawater
Abstract
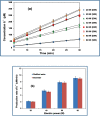
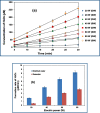
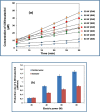
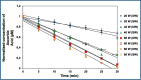
Due to the complex physical and chemical interactions taking place in the sonicated medium, various methods have been proposed in the literature for a better understanding of the sonochemical system. In the present paper, the performance of calorimetry, iodometry, Fricke, 4-nitrophenol, H2O2, and ascorbic acid dosimetry techniques have been evaluated over the electric power range from 20 to 80 W (f = 300 kHz). These methods have been analyzed for distilled and seawater in light of the literature findings. It has been found that the lowest temperatures and calorimetric energies were obtained for seawater in comparison to distilled water. However, the discrepancy between both mediums disappears with the increase in the electric power up to 80 W. Compared to the calorimetry results, a similar trend was obtained for the KI dosimetry, where the discrepancy between both solutions (seawater and distilled water) increased with the reduction in the electric power down to 20 W. In contrast, over the whole range of the electric power (20-80 W), the H2O2 dosimetry was drastically influenced by the salt composition of seawater, where, I3- formation was clearly reduced in comparison to the case of the distilled water. On the other hand, a fluctuated behavior was observed for the Fricke and 4-nitrophenol dosimetry methods, especially at the low electric powers (20 and 40 W). It has been found that dosimetry techniques based on ascorbic acid or potassium iodide are the best means for accurate quantification of the sonochemical activity in the irradiated liquid. As a result, it has been concluded, in terms of the dosimetry process's performance, that the dosimetry methods are in the following order: Ascorbic acid ≈ KI > Fricke > 4-nitrophenol > H2O2.
Keywords: 4-Nitrophenol; Ascorbic acid; Dosimetry; Fricke; H(2)O(2); Iodometry.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Yao Y., Chen Q., Huang Z., Zhou J. Catalytic activity comparison of typical iron-bearing particle electrodes in heterogeneous electro-Fenton oxidation processes. Environ. Technol. Innov. 2021;21:101321. doi: 10.1016/j.eti.2020.101321. - DOI
-
- Meghlaoui F.Z., Merouani S., Hamdaoui O., Bouhelassa M., Ashokkumar M. Rapid catalytic degradation of refractory textile dyes in Fe(II)/chlorine system at near neutral pH: Radical mechanism involving chlorine radical anion (Cl2°−)-mediated transformation pathways and impact of environmental matrices. Sep. Purif. Technol. 2019;227 doi: 10.1016/j.seppur.2019.115685. - DOI
-
- Villegas-Guzman P., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O., Petrier C., Torres-Palma R.A. Enhancement and inhibition effects of water matrices during the sonochemical degradation of the antibiotic dicloxacillin. Ultrason. Sonochem. 2015;22:211–219. doi: 10.1016/j.ultsonch.2014.07.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

