Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury
- PMID: 37944523
- PMCID: PMC10922137
- DOI: 10.1016/j.chembiol.2023.10.012
Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury
Abstract
Iron overload, characterized by accumulation of iron in tissues, induces a multiorgan toxicity whose mechanisms are not fully understood. Using cultured cell lines, Caenorhabditis elegans, and mice, we found that ferroptosis occurs in the context of iron-overload-mediated damage. Exogenous oleic acid protected against iron-overload-toxicity in cell culture and Caenorhabditis elegans by suppressing ferroptosis. In mice, oleic acid protected against FAC-induced liver lipid peroxidation and damage. Oleic acid changed the cellular lipid composition, characterized by decreased levels of polyunsaturated fatty acyl phospholipids and decreased levels of ether-linked phospholipids. The protective effect of oleic acid in cells was attenuated by GW6471 (PPAR-α antagonist), as well as in Caenorhabditis elegans lacking the nuclear hormone receptor NHR-49 (a PPAR-α functional homologue). These results highlight ferroptosis as a driver of iron-overload-mediated damage, which is inhibited by oleic acid. This monounsaturated fatty acid represents a potential therapeutic approach to mitigating organ damage in iron overload individuals.
Keywords: C. elegans; MUFA; PPAR; cancer; cell death; degeneration; ferroptosis; iron; lipid; olei acid.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests B.R.S. is an inventor on patents and patent applications involving ferroptosis, holds equity in and serves as a consultant to Exarta Therapeutics, and ProJenX Inc, holds equity in Sonata Therapeutics, and serves as a consultant to Weatherwax Biotechnologies Corporation and Akin Gump Strauss Hauer & Feld LLP.
Figures

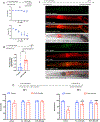


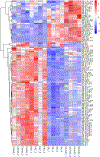

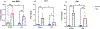
References
-
- Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, Trenor CC, Gasparini P, Andrews NC, and Pietrangelo A (2001). Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Invest 108, 619–623. 10.1172/JCI200113468. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

