Intravenous immunoglobulin treatment in childhood encephalitis (IgNiTE): a randomised controlled trial
- PMID: 37945292
- PMCID: PMC10649701
- DOI: 10.1136/bmjopen-2023-072134
Intravenous immunoglobulin treatment in childhood encephalitis (IgNiTE): a randomised controlled trial
Abstract
Objective: To investigate whether intravenous immunoglobulin (IVIG) improves neurological outcomes in children with encephalitis when administered early in the illness.
Design: Phase 3b multicentre, double-blind, randomised placebo-controlled trial.
Setting: Twenty-one hospitals in the UK.
Participants: Children aged 6 months to 16 years with a diagnosis of acute or subacute encephalitis, with a planned sample size of 308.
Intervention: Two doses (1 g/kg/dose) of either IVIG or matching placebo given 24-36 hours apart, in addition to standard treatment.
Main outcome measure: The primary outcome was a 'good recovery' at 12 months after randomisation, defined as a score of≤2 on the Paediatric Glasgow Outcome Score Extended.
Secondary outcome measures: The secondary outcomes were clinical, neurological, neuroimaging and neuropsychological results, identification of the proportion of children with immune-mediated encephalitis, and IVIG safety data.
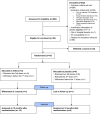
Results: 18 participants were recruited from 12 hospitals and randomised to receive either IVIG (n=10) or placebo (n=8) between 23 December 2015 and 26 September 2017. The study was terminated early following withdrawal of funding due to slower than anticipated recruitment, and therefore did not reach the predetermined sample size required to achieve the primary study objective; thus, the results are descriptive. At 12 months after randomisation, 9 of the 18 participants (IVIG n=5/10 (50%), placebo n=4/8 (50%)) made a good recovery and 5 participants (IVIG n=3/10 (30%), placebo n=2/8 (25%)) made a poor recovery. Three participants (IVIG n=1/10 (10%), placebo n=2/8 (25%)) had a new diagnosis of epilepsy during the study period. Two participants were found to have specific autoantibodies associated with autoimmune encephalitis. No serious adverse events were reported in participants receiving IVIG.
Conclusions: The IgNiTE (ImmunoglobuliN in the Treatment of Encephalitis) study findings support existing evidence of poor neurological outcomes in children with encephalitis. However, the study was halted prematurely and was therefore underpowered to evaluate the effect of early IVIG treatment compared with placebo in childhood encephalitis.
Trial registration number: Clinical Trials.gov NCT02308982; ICRCTN registry ISRCTN15791925.
Keywords: clinical trials; developmental neurology & neurodisability; paediatric infectious disease & immunisation; paediatric neurology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MI was a trainee member on the NIHR Efficacy and Mechanism Evaluation Programme Funding Committee from October 2020 to October 2021. MA has received a grant from the NIHR in the last 36 months, for research unrelated to the submitted work. MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines; all funds have been paid to his institute. Ava Easton is Chief Executive of the Encephalitis Society, which has previously received grants from CSL Behring (UK). Ming Lim has received grants from the GOSH charity, Boston Children’s Hospital Research Fund and Action Medical Research in the last 36 months, all for research unrelated to the submitted work. Ming Lim is co-chair of the European Paediatric Neurology Education and Training Board and works for an institution which holds research accounts with Roche (Switzerland), Octapharma (Switzerland) and Novartis (Switzerland). Tom Solomon is supported by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections, NIHR Programme Grant for Applied Research, NIHR Global Health Research on Brain Infections and the European Union’s Horizon 2020 research and innovation program ZikaPLAN. Tom Solomon is a consultant for the MHRA Vaccine Benefit Risk Expert Working Group. Angela Vincent is a consultant for Aspen NewCo Inc and has received honoraria from UCB and Alexion. L-MY was a member of the NIHR Health Technology Assessment Efficient Study Designs from November 2015 to July 2016. Andrew J Pollard is chair of the Department of Health and Social Care’s Joint committee on Vaccines and Immunisation (JCVI) and was a member of WHO’s SAGE until 1 January 2022. Oxford University has entered a partnership with AstraZenenca on COVID-19 vaccines, but Andrew Pollard does not participate in the JCVI COVID-19 committee.
Figures
References
-
- Dr Julia Granerod AE, Davies DN, Michael DB, et al. Encephalitis: an in-depth review and gap analysis of key variables affecting global disease burden. Encephalitis Society 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical