A Phase 2 Trial of Talazoparib and Avelumab in Genomically Defined Metastatic Kidney Cancer
- PMID: 37945488
- PMCID: PMC11074239
- DOI: 10.1016/j.euo.2023.10.017
A Phase 2 Trial of Talazoparib and Avelumab in Genomically Defined Metastatic Kidney Cancer
Abstract
Background: Although different kidney cancers represent a heterogeneous group of malignancies, multiple subtypes including Von Hippel-Lindau (VHL)-altered clear cell renal cell carcinoma (ccRCC), fumarate hydratase (FH)- and succinate dehydrogenase (SDH)-deficient renal cell carcinoma (RCC), and renal medullary carcinoma (RMC) are affected by genomic instability. Synthetic lethality with poly ADP-ribose polymerase inhibitors (PARPis) has been suggested in preclinical models of these subtypes, and paired PARPis with immune checkpoint blockade (ICB) may achieve additive and/or synergistic effects in patients with previously treated advanced kidney cancers.
Objective: To evaluate combined PARPi + ICB in treatment-refractory metastatic kidney cancer.
Design, setting, and participants: We conducted a single-center, investigator-initiated phase 2 trial in two genomically selected advanced kidney cancer cohorts: (1) VHL-altered RCC with at least one prior ICB agent and one vascular endothelial growth factor (VEGF) inhibitor, and (2) FH- or SDH-deficient RCC with at least one prior ICB agent or VEGF inhibitor and RMC with at least one prior line of chemotherapy.
Intervention: Patients received talazoparib 1 mg daily plus avelumab 800 mg intravenously every 14 d in 28-d cycles.
Outcome measurements and statistical analysis: The primary endpoint was objective response rate (ORR) by Immune Response Evaluation Criteria in Solid Tumors at 4 mo, and the secondary endpoints included progression-free survival (PFS), overall survival, and safety.
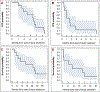
Results and limitations: Cohort 1 consisted of ten patients with VHL-altered ccRCC. All patients had previously received ICB. The ORR was 0/9 patients; one patient was not evaluable due to missed doses. In this cohort, seven patients achieved stable disease (SD) as the best response. The median PFS was 3.5 mo (95% confidence interval [CI] 1.0, 3.9 mo). Cohort 2 consisted of eight patients; four had FH-deficient RCC, one had SDH-deficient RCC, and three had RMC. In this cohort, six patients had previously received ICB. The ORR was 0/8 patients; two patients achieved SD as the best response and the median PFS was 1.2 mo (95% CI 0.4, 2.9 mo). The most common treatment-related adverse events of all grades were fatigue (61%), anemia (28%), nausea (22%), and headache (22%). There were seven grade 3-4 and no grade 5 events.
Conclusions: The first clinical study of combination PARPi and ICB therapy in advanced kidney cancer did not show clinical benefit in multiple genomically defined metastatic RCC cohorts or RMC.
Patient summary: We conducted a study to look at the effect of two medications, talazoparib and avelumab, in patients with metastatic kidney cancer who had disease progression on standard treatment. Talazoparib blocks the normal activity of molecules called poly ADP-ribose polymerase, which then prevents tumor cells from repairing themselves and growing, while avelumab helps the immune system recognize and kill cancer cells. We found that the combination of these agents was safe but not effective in specific types of kidney cancer.
Keywords: Fumarate hydratase deficient; Immunotherapy; Metastatic; Poly ADP-ribose polymerase inhibitor; Programmed death-ligand 1; Renal cell carcinoma; Renal medullary cancer; SMARCB1; Succinate dehydrogenase deficient; Von Hippel-Lindau altered.
Copyright © 2023 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- National Comprehensive Cancer Network. Kidney cancer (version 4.2023). https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
-
- Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma [correction, Cell Rep. 2018 Jun 19;23:3698]. Cell Rep 2018;23:313–326.e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

