Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation
- PMID: 37945752
- PMCID: PMC11310082
- DOI: 10.1038/s41551-023-01113-2
Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation
Erratum in
-
Author Correction: Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation.Nat Biomed Eng. 2024 Nov;8(11):1501. doi: 10.1038/s41551-024-01200-y. Nat Biomed Eng. 2024. PMID: 38565945 Free PMC article. No abstract available.
Abstract
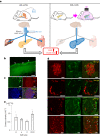
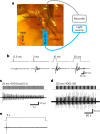
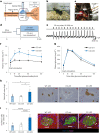
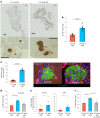
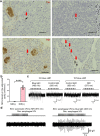
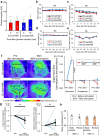
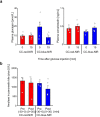
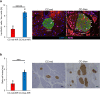
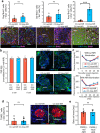
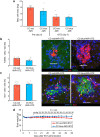
The enhancement of insulin secretion and of the proliferation of pancreatic β cells are promising therapeutic options for diabetes. Signals from the vagal nerve regulate both processes, yet the effectiveness of stimulating the nerve is unclear, owing to a lack of techniques for doing it so selectively and prolongedly. Here we report two optogenetic methods for vagal-nerve stimulation that led to enhanced glucose-stimulated insulin secretion and to β cell proliferation in mice expressing choline acetyltransferase-channelrhodopsin 2. One method involves subdiaphragmatic implantation of an optical fibre for the photostimulation of cholinergic neurons expressing a blue-light-sensitive opsin. The other method, which suppressed streptozotocin-induced hyperglycaemia in the mice, involves the selective activation of vagal fibres by placing blue-light-emitting lanthanide microparticles in the pancreatic ducts of opsin-expressing mice, followed by near-infrared illumination. The two methods show that signals from the vagal nerve, especially from nerve fibres innervating the pancreas, are sufficient to regulate insulin secretion and β cell proliferation.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures



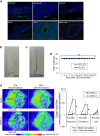
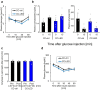
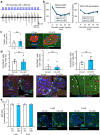
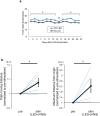
References
Publication types
MeSH terms
Substances
Grants and funding
- 21gm6210002h0004/Japan Agency for Medical Research and Development (AMED)
- JP21gm5010002h0005/Japan Agency for Medical Research and Development (AMED)
- 22K19303/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K17525/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20H05694/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical

